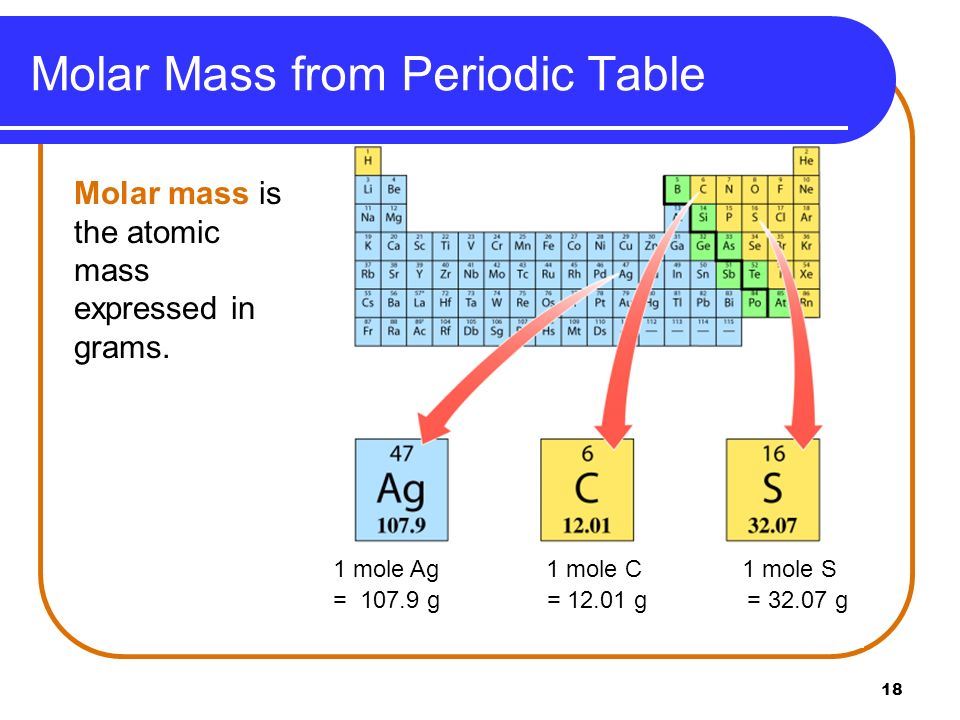
mole vs molar mass For an element the molar mass is the mass of 1 mol of atoms of that element for a covalent molecular compound it is the mass of 1 mol of molecules of that compound for an ionic compound it is the mass of 1 mol of formula units
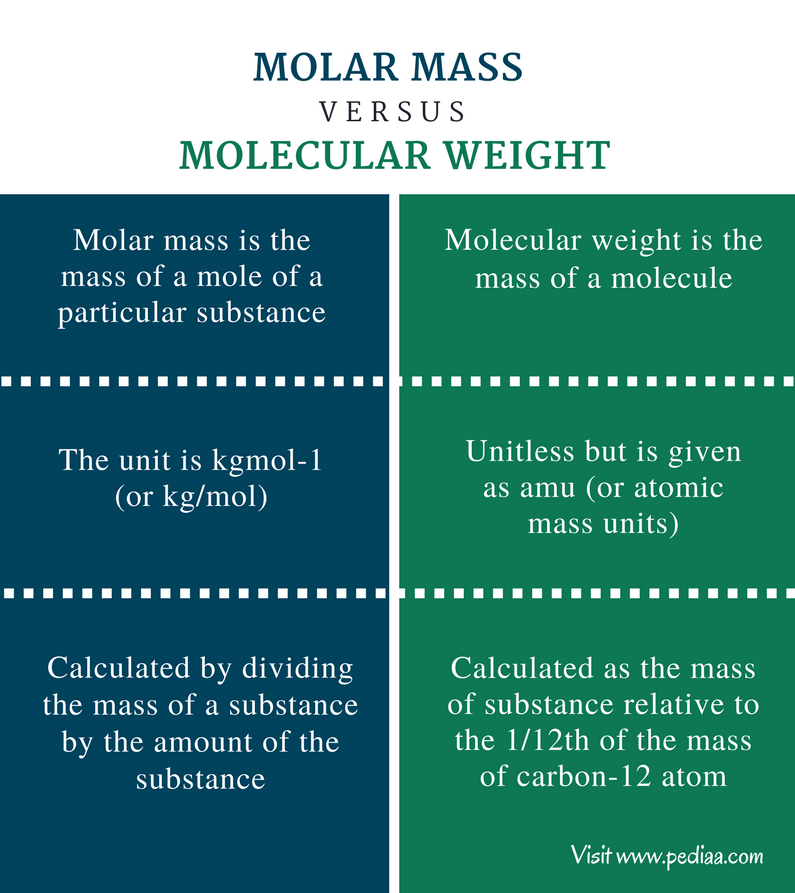
In chemistry the molar mass M sometimes called molecular weight or formula weight but see related quantities for usage of a chemical compound is defined as the ratio between the mass and the amount of substance measured in moles of any sample of the compound The molar mass is a bulk not molecular property of a substance The molar mass is an average of many instances of th The molar mass of a substance is the molecular weight but it is expressed in grams not in amu All you need to do is calculate the molecular weight and stick the unit g mol grams per
mole vs molar mass
mole vs molar mass
https://cdn1.byjus.com/chemistry/wp-content/uploads/2016/01/slide_18.jpg

Difference Between Molar Mass And Molecular Weight Definition
http://pediaa.com/wp-content/uploads/2017/06/Difference-Between-Molar-Mass-and-Molecular-Weight-3.png
Mole Mass Conversions
https://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/section_09/9e62b1f06d79342c219139c91ea771be.jpg
For an element the molar mass is the mass of 1 mol of atoms of that element for a covalent molecular compound it is the mass of 1 mol of molecules of that compound for Molar mass is the mass of one mole of a substance and is expressed in grams per mole text g mol g mol Chemists denote molar mass with the symbol text M M It is a useful quantity in chemistry because it
What is the mole and Avogadro s constant The mole is the unit for amount of substance The number of particles in a substance can be found using the Avogadro constant The mass of This Khan Academy page provides a worked example of calculating molar mass and the number of moles
More picture related to mole vs molar mass
Difference Between Molar Mass And Molecular Weight Definition
https://pediaa.com/wp-content/uploads/2017/06/Difference-Between-Molar-Mass-and-Molecular-Weight-Comparison-Summary.png

Experiment 14 Lab Report Chem Molar Mass Of Of A Solid Molar Mass Of
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/27fd2f7e551adcc8b11446553cd48475/thumb_1200_1553.png

Thermal DP Physics IB Recap
http://2.bp.blogspot.com/-g8bPaD78cow/VYbiULFQFRI/AAAAAAAABpI/EgrJShn2S54/s1600/molar_conversion.jpg
In chemistry the molar mass is the mass in grams per mole g mol or kilograms per mole kg mol of a substance Molar mass is an intensive property of matter meaning its value does not depend on sample size Follow Molar mass of a substance is the mass in grams of one mole of the compound In a substance the amount of entities present e g atoms molecules ions is defined as a mole A
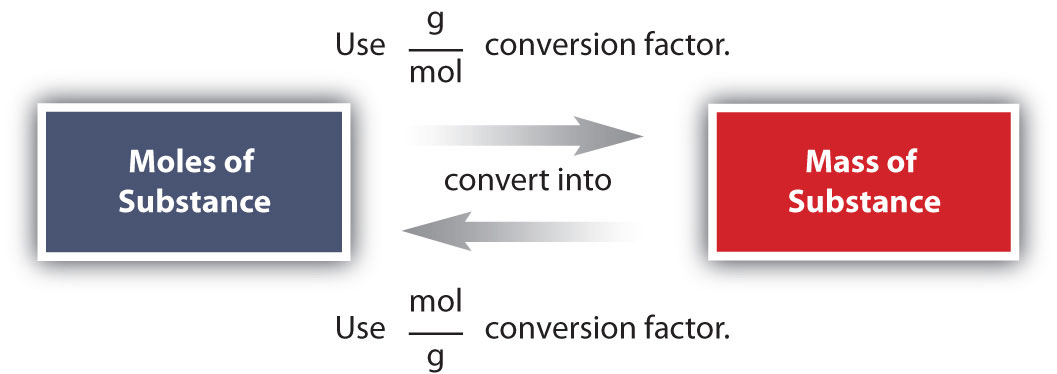
Conversions Between Moles and Mass The molar mass of any substance is the mass in grams of one mole of representative particles of that substance The representative particles can The molar mass is the mass of 1 mol of this atom If you know the molar mass of an element you can calculate the mass of any sample of this element provided you
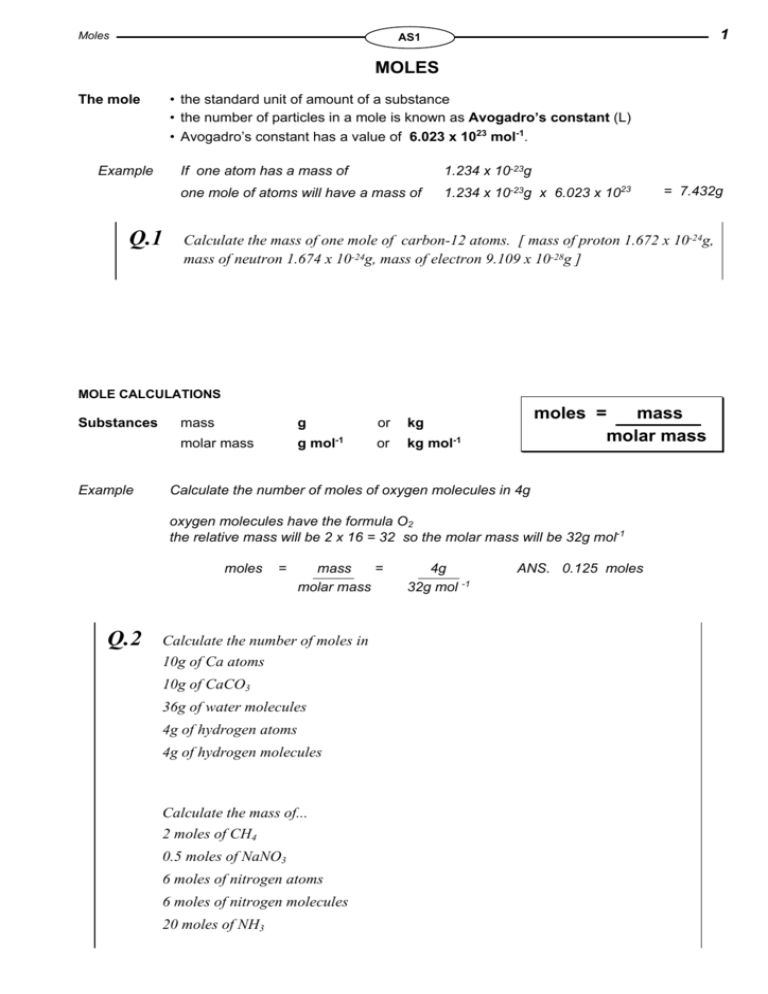
MOLES Moles Mass Molar Mass
https://s3.studylib.net/store/data/008195363_1-7ef902d1ee11a899bf3b4e7bcdd7a818-768x994.png

Mole Road Map Overview Examples Of Conversions Expii
https://d20khd7ddkh5ls.cloudfront.net/img_b9f266de9b7f-1.jpeg
mole vs molar mass - For an element the molar mass is the mass of 1 mol of atoms of that element for a covalent molecular compound it is the mass of 1 mol of molecules of that compound for