difference between mole and molar mass For an element the molar mass is the mass of 1 mol of atoms of that element for a covalent molecular compound it is the mass of 1 mol of molecules of that compound for an ionic compound it is the mass of 1 mol of formula units
For an element the molar mass is the mass of 1 mol of atoms of that element for a covalent molecular compound it is the mass of 1 mol of molecules of that compound for In chemistry the molar mass M sometimes called molecular weight or formula weight but see related quantities for usage of a chemical compound is defined as the ratio between the mass and the amount of substance measured in moles of any sample of the compound The molar mass is a bulk not molecular property of a substance The molar mass is an average of many instances of th
difference between mole and molar mass

difference between mole and molar mass
https://i.ytimg.com/vi/OXXuHg-S2QY/maxresdefault.jpg
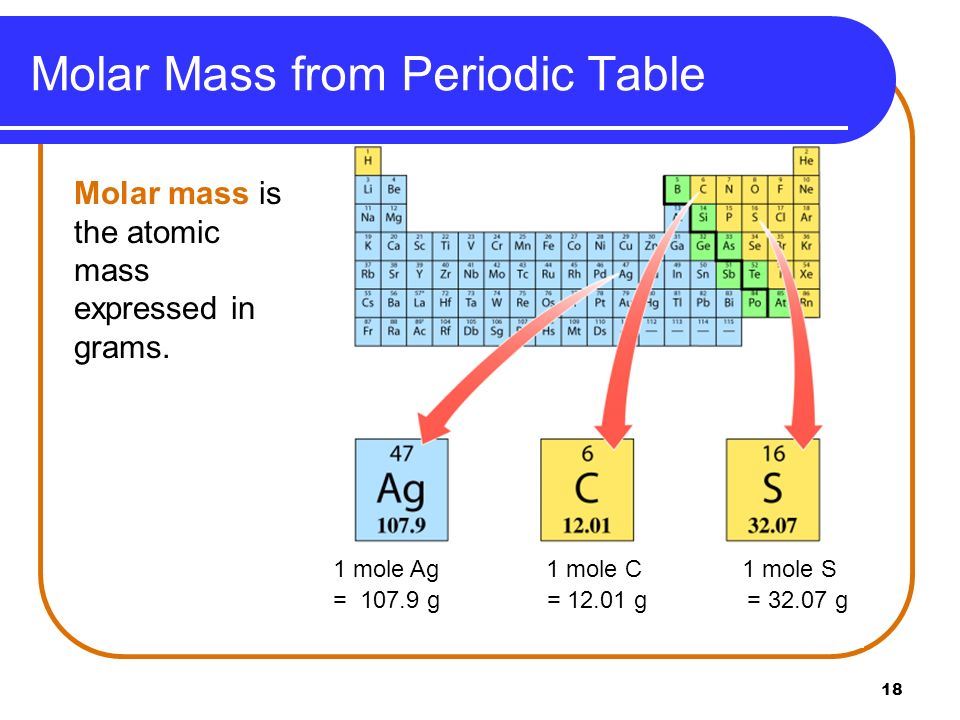
Atomic Mass And Molecular Mass Definition Difference Mass Spectrometry
https://cdn1.byjus.com/chemistry/wp-content/uploads/2016/01/slide_18.jpg
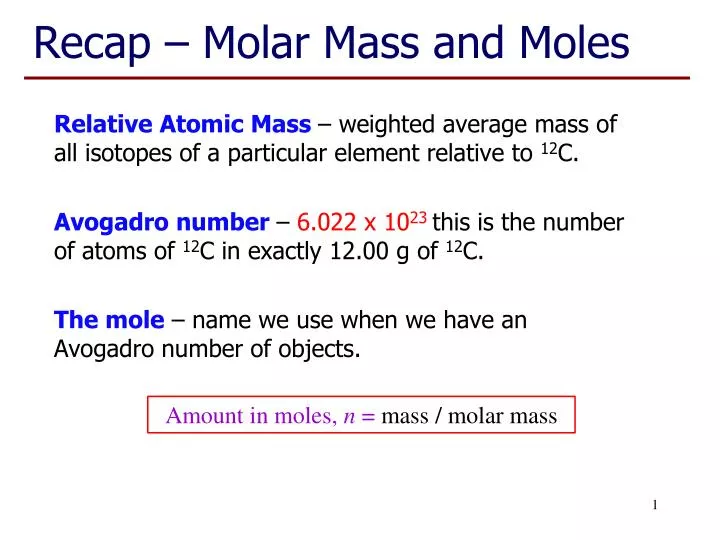
PPT Recap Molar Mass And Moles PowerPoint Presentation Free
https://image1.slideserve.com/2567957/recap-molar-mass-and-moles-n.jpg
The given mass is the mass of the sample and it can be any number for example we can have 10 g of salt 15 g or 100 g The molar mass on the other hand is a constant number for a given atom or a molecule as it is for a The molar mass is the mass of 1 mol of this atom If you know the molar mass of an element you can calculate the mass of any sample of this element provided you
Molarity is expressed in moles per liter mol L or M while the mole is expressed in moles mol Molarity provides a concentration value whereas the mole represents a specific quantity of a Molar mass is the mass of one mole of a substance and is expressed in grams per mole text g mol Chemists denote molar mass with the symbol text M It is a useful quantity in chemistry because it allows us to
More picture related to difference between mole and molar mass

What Is Mole What Is The Difference Between Molecular Mass Molar
https://i.ytimg.com/vi/YfkDdcV1f-M/maxresdefault.jpg
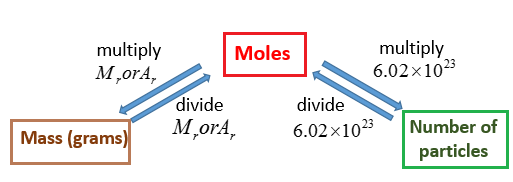
Molar Mass Grams Moles solutions Examples Activities Experiment
https://www.onlinemathlearning.com/image-files/xmole-mass-avogadro.png.pagespeed.ic.QfUP0vcyUg.png
Molar Mass Vs Molecular Mass Definition And 6 Key Differences
https://thechemistrynotes.com/wp-content/uploads/2021/05/Molar-Mass-vs-Molecular-Mass.jpeg
Molecules and atoms are very tiny both in size and mass The molar mass is the weight of one sample mole Connect the atomic masses atomic weights of all atoms within the molecule to The masses of 1 mole of different elements however are different since the masses of the individual atoms are drastically different The molar mass of an element or compound is the
The difference between mole and molar mass is that the number of molecules used to produce one mole of any substance is constant Different molecules however would have Molar mass refers to the mass of one mole of a substance which could be an element or a compound The molar mass of an element in terms of atom is equal to its relative atomic

How Do I Find The Moles And Molar Mass Of An Unknown Solid Acid YouTube
https://i.ytimg.com/vi/w-RQfUnuzvU/maxresdefault.jpg
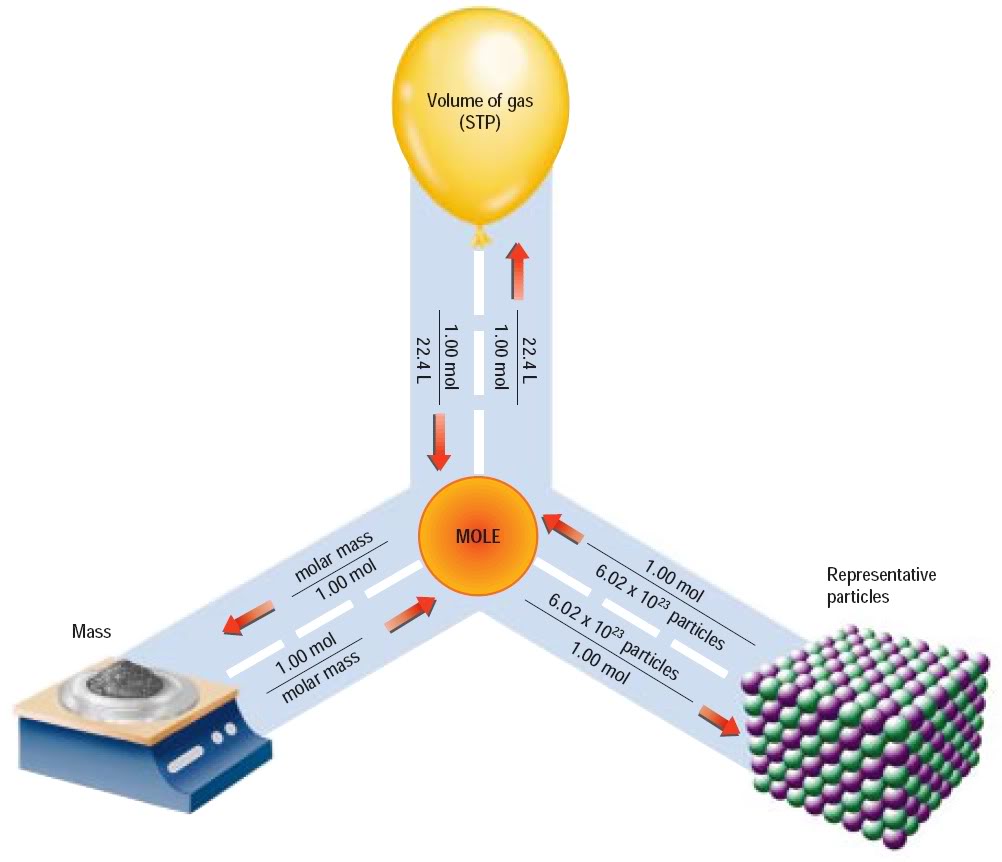
Chemistry Time Converting Between Volume And Moles
http://1.bp.blogspot.com/-hrCm30OOPVg/TuUGIPFphXI/AAAAAAAAAFE/ePAPtZAIoK0/s1600/Mole+Relationships.jpg
difference between mole and molar mass - Firstly atomic mass refers to the mass of an individual atom whereas molar mass represents the mass of one mole of a substance Atomic mass is specific to an element while molar