human factors medical device guidance This guidance provides a risk based framework to guide stakeholders on the human factors information that should be included in a marketing submission to CDRH
Why is Human Factor Engineering important to medical devices For medical devices the most important goal of the human factors usability engineering process is to minimize This guidance focuses on ways in which human factors can be applied to medical devices so that they are designed and optimised for use by intended users in the environment in which
human factors medical device guidance

human factors medical device guidance
https://www.mindflowdesign.com/wp-content/uploads/2019/11/img_5968-r1-1024x768.jpg
Human Factors Engineering For Medical Devices EMMA International
https://emmainternational.com/wp-content/uploads/2021/01/HFE-Med-Devices_Blog-01-768x512.jpg

FDA Human Factors Guidance Framework For Human Factors Information In
https://operonstrategist.com/wp-content/uploads/2022/12/FDA-human-factor-guidance-01-1536x803.jpg
A large proportion of the patient injuries or deaths attributable to medical device MD misuse can be eliminated and or mitigated by adopting an effective human factors and This article focuses on the human factors of those medical devices a significant cause of adverse events in the ICU Keywords Adverse events Human factors Intensive care unit
Guidance on applying human factors to medical devices Updated 12 February 2021 Download CSV 533 KB This CSV cannot be viewed online You can download the file to open A large proportion of the patient injuries or deaths attributable to medical device MD misuse can be eliminated and or mitigated by adopting an effective human factors and
More picture related to human factors medical device guidance
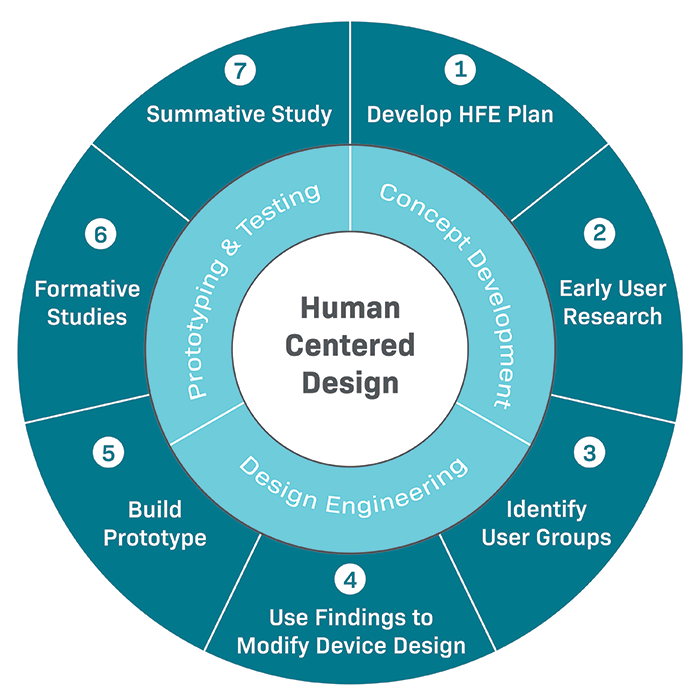
The Importance Of Human Factors For Medical Devices Gilero
https://www.gilero.com/wp-content/uploads/2020/04/Human-Factors-Infographic-1.png

FDA Medical Device Guidance Substantial Equivalence
https://cohenhealthcarelaw.com/michaelhcohen/wp-content/uploads/2019/02/bigstock-various-colour-pills-and-medic-4988625.jpg

Applying Human Factors And Usability Engineering To Medical Devices
https://sterlingmedicaldevices.com/app/uploads/2021/11/applying-human-factors-and-usability-engineering-to-medical-1.png
FDA has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to maximize the likelihood that Human factors and usability engineering processes to maximize the likelihood that new medical devices will be safe and effective for the intended users uses and use
This guideline is a general requirement for the human factors design of medical devices Manufacturers should determine the applicability of specific elements of this guidance based This guidance will focus on ways in which human factors ergonomics and usability engineering can be applied to medical devices so that they are designed and optimised for use by

An Introduction To Human Factors Engineering In Medical Device Design
https://arrotek.com/wp-content/uploads/2021/05/An-Introduction-to-Human-Factors-Engineering-in-Medical-Device-Design.jpg

A Short Guide To Human Factors In Medical Devices Educo Life Sciences
https://educolifesciences.com/wp-content/uploads/2021/01/Blog-Pst-800x533-1-1.jpg
human factors medical device guidance - FDA s most recent guidance on human factors Applying Human Factors and Usability Engineering to Medical Devices highlights the importance of enhancing patient