human factors principles for medical device labeling State general warnings about device use at an early point in the labeling In contrast state specific warnings in the appropriate procedure sections just before the step to which they
Human Factors Usability Validation Demonstrates and provides evidence that a medical device as designed can be used safely and effectively Human Factors Validation Demonstrates and provides evidence that a medical device as designed can be used safely and effectively
human factors principles for medical device labeling

human factors principles for medical device labeling
https://reader038.dokumen.tips/reader038/viewer/2023022218/63209073e9691360fe01d7dd/html5/page/1.jpg

Understanding Human Factors For Medical Devices Johari Digital
https://www.joharidigital.com/wp-content/uploads/2022/02/human-factors-of-medical-devices.jpg

PDF Human Factors Principles For Medical Device Labeling DOKUMEN TIPS
https://reader038.dokumen.tips/reader038/viewer/2023022218/63209073e9691360fe01d7dd/html5/page/7.jpg
ABSTRACT Developed to promote the design of safe effective and usable medical devices Handbook of Human Factors in Medical Device Design provides a single convenient source of authoritative information to This article focuses on the human factors of those medical devices a significant cause of adverse events in the ICU Keywords Adverse events Human factors Intensive care unit
Medical devices Application of usability engineering to medi cal devices The companion document ANSI AAMI HE75 2009 is the committee s effort to provide comprehensive hu man One part of this would be to design evaluate and validate medical devices with human factors and ergonomics principles in mind
More picture related to human factors principles for medical device labeling

Tangents Introduction To Human Factors For Medical Devices
https://assets-global.website-files.com/5ec3efa8b380ded8f9609b83/6079afbc022347d010d53ac2_Domain of Human Factors.png
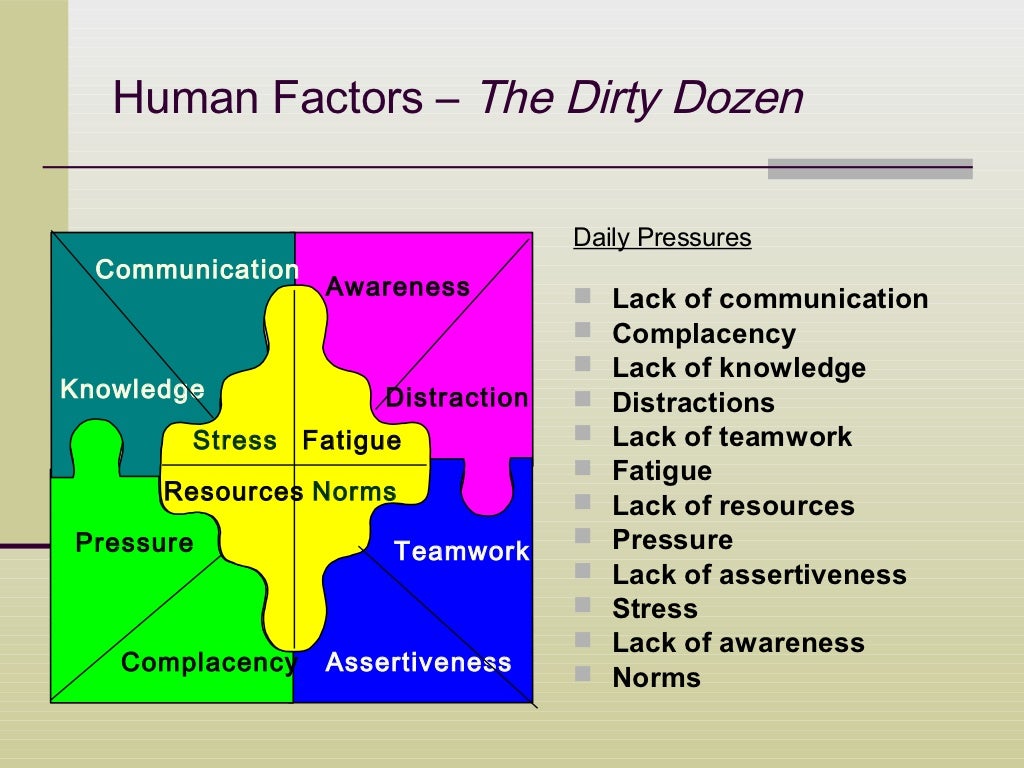
Human Factors
https://image.slidesharecdn.com/humanfactors-120823171049-phpapp01/95/slide-8-1024.jpg
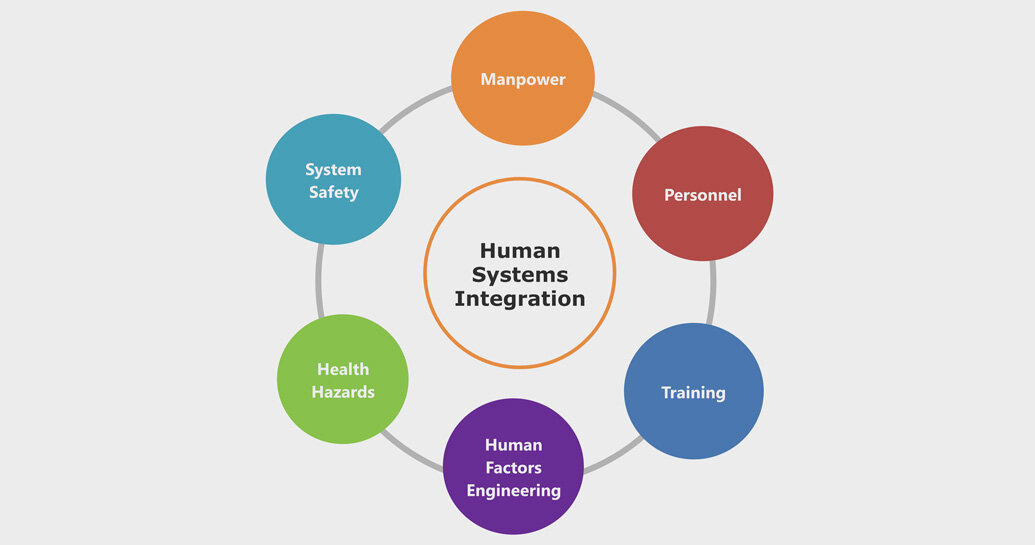
What Is Human Factors Design Talk
https://images.squarespace-cdn.com/content/v1/5dca320893240c1f890584b4/1590369130017-IVMSR9WHM8GHYM03SC0N/Erg_HFI_04.jpg
In this article we initially discuss human factors as defined by the FDA followed by three classic case studies The ramifications of legal issues are then presented Concurrent Medical device labeling consists of directions on how to use and care for medical devices as well as information necessary for ensuring users understanding and safety including information about risks precautions and
Primer discusses human factors problems general design principles and human factors engineering methods and uses examples and illustrations for clarification The Food and Drug FDA has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to maximize the likelihood that new medical
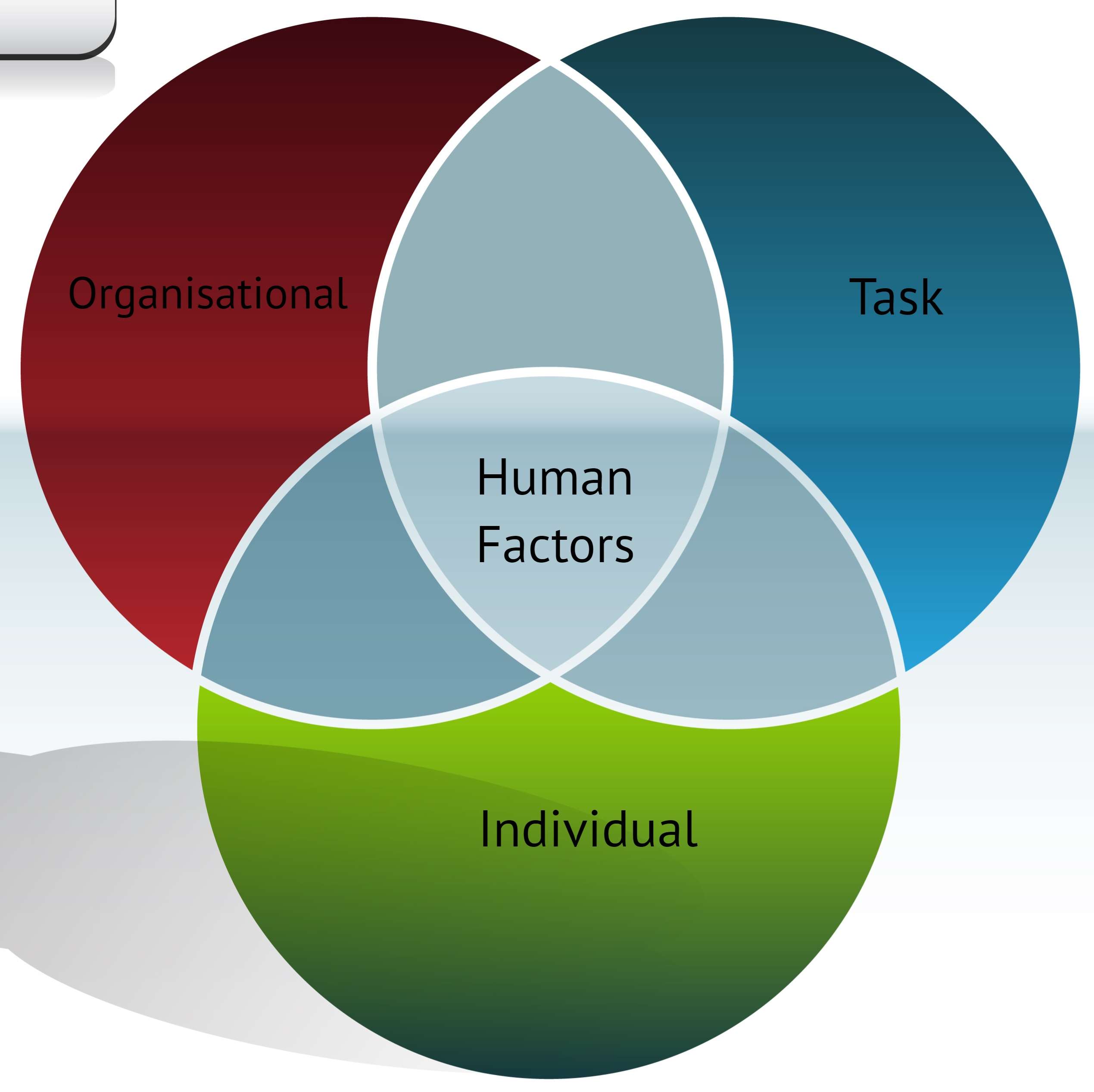
8 Principles Of Manual Handling Compliplus
https://compliplus.com/wp-content/uploads/2021/07/Compliplus_human_factors_health_safety-1.jpg
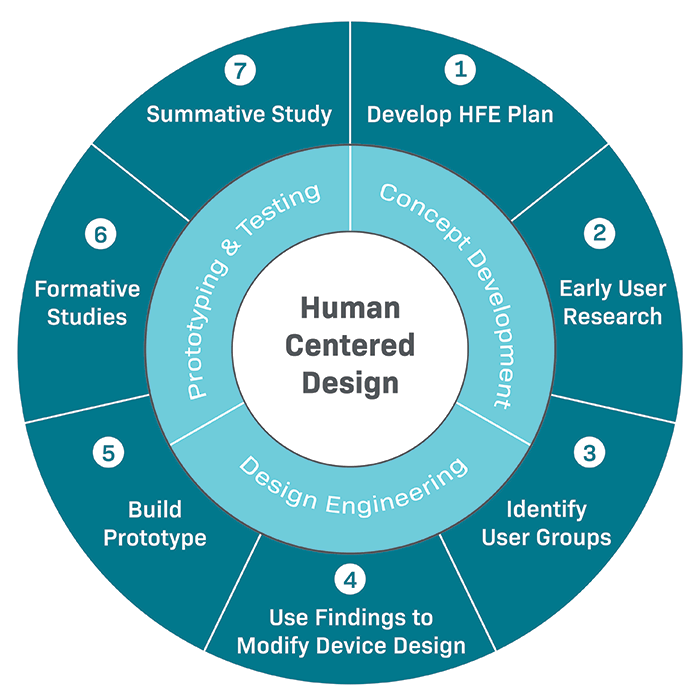
The Importance Of Human Factors For Medical Devices Gilero
https://www.gilero.com/wp-content/uploads/2020/04/Human-Factors-Infographic.png
human factors principles for medical device labeling - Medical devices Application of usability engineering to medi cal devices The companion document ANSI AAMI HE75 2009 is the committee s effort to provide comprehensive hu man