34 protons what element is it 119 rowsHere is a list of elements of the periodic table their
The number of protons in an atom is called its atomic number Z Z This number is very important because it is unique for atoms of a given element All atoms of an element have the same The number of protons is the atomic number and is what distinguishes an element from another element The atomic mass or mass number corresponds to the mass of the nucleus and
34 protons what element is it
34 protons what element is it
https://lh6.googleusercontent.com/proxy/cHmJ4HVruNArIS-g6hNX89WoA7T5gTJJW20eAPyow--172JAhLyOQCyxKdJr3UfUzF_joaggZotDXoiZhW_KT36u-nz_BwYXA2Ja7QWs2wxcbBSZT9x-3qxMsVQkRRJbVs5MypfI8nI84w=s0-d

Protons Neutrons And Subatomic Particles Pharmacy Gyan
https://pharmacygyan.com/wp-content/uploads/2021/09/protons.jpeg
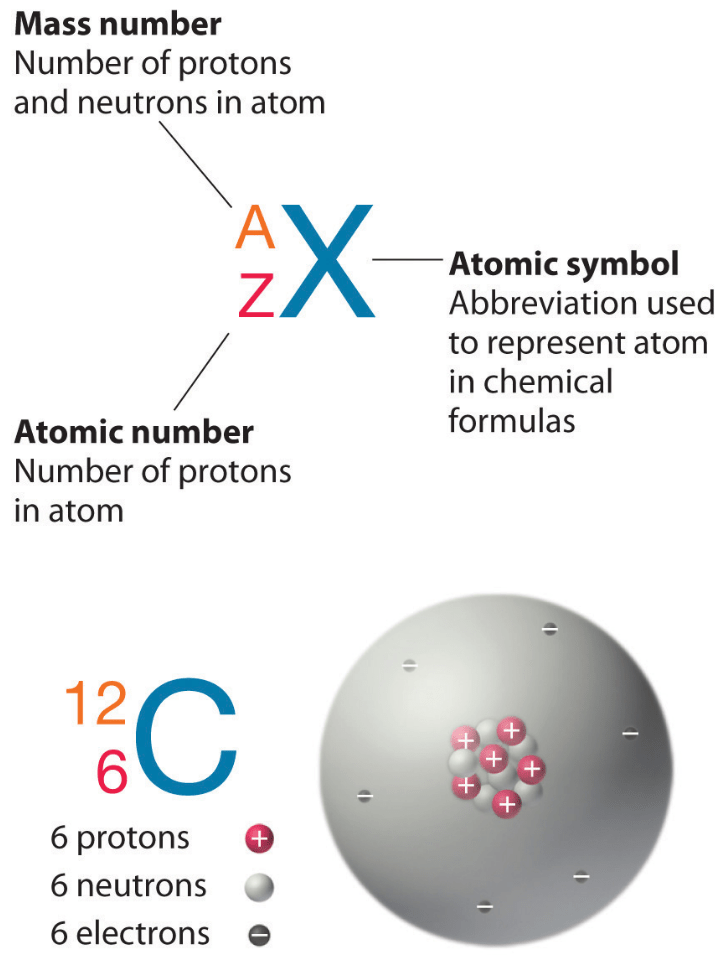
Atomic Number Mass Number Isotopes And Calculations ClassNotes ng
https://classnotes.ng/wp-content/uploads/2020/04/ATOMIC-NUMBER-MASS-NUMBER-ISOTOPES-AND-CALCULATIONS.png
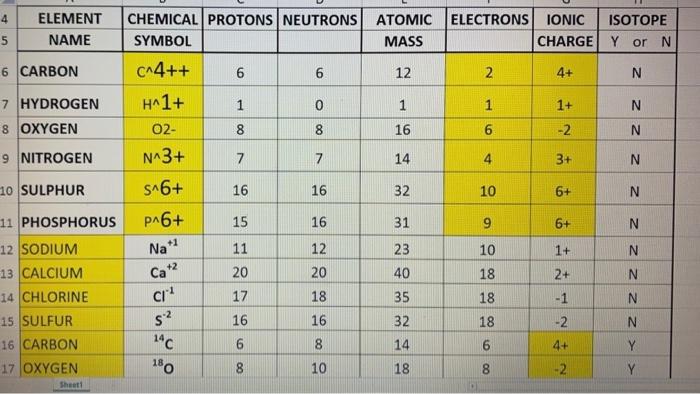
Describe the locations charges and masses of the three main subatomic particles Determine the number of protons and electrons in an atom Write and interpret symbols that From the periodic table we find that it has 29 protons The mass number 65 is the sum of the number of protons and neutrons Therefore we can subtract the number of protons from the atomic number to get the number of neutrons n
Z 34 therefore the atom is selenium While the number nuclear protons as given is 34 and therefore we deal with the element selenium there are 2 more electrons than Element Selenium Se Group 16 Atomic Number 34 p block Mass 78 971 Sources facts uses scarcity SRI podcasts alchemical symbols videos and images
More picture related to 34 protons what element is it
What Is N In The Periodic Table
http://www.sliderbase.com/images/referats/1132b/(1).PNG

Iron Protons Neutrons Electrons Electron Configuration
https://material-properties.org/wp-content/uploads/2020/09/Iron-protons-neutrons-electrons-configuration.png

Boron Atom Google Search Lasbril Auteurs Afbeeldingen
https://i.pinimg.com/originals/9d/d6/70/9dd670c51024fa6a650e237ba2793e6c.jpg
If there are 34 protons the element is unquestionably selenium Explanation The number of protons the number of massive positively charged nuclear particles is Z the So what is the element Atomic number Z 34 Z refers to the number of protons positively charged nuclear particles This number specifies the identity of the element
The symbol for the ion containing 34 protons 46 neutrons and 36 electrons is Se2 This corresponds to Selenium Se with a charge of 2 indicating that it has lost 2 electrons The number of protons in an atom or ion If the 2 ion has 34 protons it means the ion has a charge of 2 but still has 34 protons making it a germanium ion Ge2
Solved Element Name Chemical Symbol Protons Neutrons Atomic Chegg
https://media.cheggcdn.com/study/bd3/bd363afc-c9e6-4d10-b88b-4887e1a721fe/image

Selenium Periodic Table And Atomic Properties
https://material-properties.org/wp-content/uploads/2020/09/Selenium-density-atomic-number-mass-radius.png
34 protons what element is it - Describe the locations charges and masses of the three main subatomic particles Determine the number of protons and electrons in an atom Write and interpret symbols that
.PNG)