ion has 34 protons what element is it In the case of a 2 ion it means that the atom has gained 2 electrons making it negatively charged Since protons determine the atomic number and hence the identity of the element an atom with 34 protons would be germanium Ge Therefore the element represented by a 2 ion with 34 protons is germanium Ge
That s where atomic number and mass number are useful Figure 4 5 1 4 5 1 It is difficult to find qualities that differ between each element and to distinguish one element from another Each element however does have a unique number of protons Sulfur has 16 protons silicon has 14 protons and gold has 79 protons Give the symbol and name for the ion with 34 protons and 36 electrons Answer Se 2 the selenide ion
ion has 34 protons what element is it

ion has 34 protons what element is it
https://i2.wp.com/4.bp.blogspot.com/-snsJdrH7YSU/VYOiYWFAUoI/AAAAAAAABe8/SP3ZPafm-G4/s1600/3.jpg
P N E Counting Jennifer Richardson Library Formative
https://d3rqz33hmpm7kb.cloudfront.net/2020-10-14/5f32df7a6fd0db82f3e8926b/5f877dc7e0427099c3a0b692_ptable.JPG
Periodic Table Of Elements List With Protons Neutrons And Electrons
https://lh6.googleusercontent.com/proxy/cHmJ4HVruNArIS-g6hNX89WoA7T5gTJJW20eAPyow--172JAhLyOQCyxKdJr3UfUzF_joaggZotDXoiZhW_KT36u-nz_BwYXA2Ja7QWs2wxcbBSZT9x-3qxMsVQkRRJbVs5MypfI8nI84w=s0-d
2 00 Ion unequal amount of protons and electrons in an atom As mentioned above there are positive and negative ions Anion negatively charged ion Cation positively charged ion Here is my way of remembering which charge is with anion and cation A in Anion becomes before C Cation in the alphabet Sodium ion on right has 17 protons and 18 electrons with a 1 overall charge The names for positive and negative ions are pronounced CAT eye ons and ANN eye ons respectively In many cases elements that belong to the same group vertical column on the periodic table form ions with the same charge because they have the
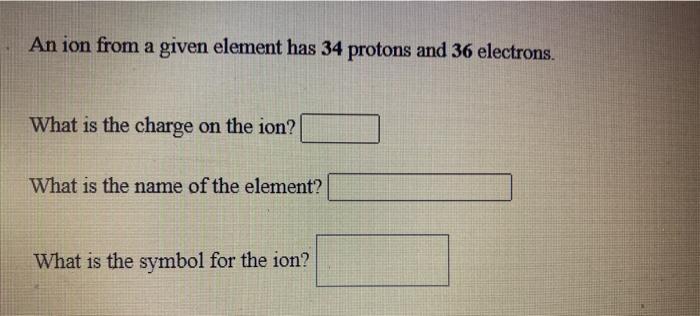
An ion from a given element has 34 protons and 36 electrons What is the charge on the ion What is the name of the element What is the symbol for the ion This problem has been solved You ll get a detailed solution from a subject matter expert that helps you learn core concepts See Answer It should be of protons of electronsIt gives you the ion charge of the element For example Oxygen ion has 8 protons and 10 electrons Therefore 8 10 2 O2
More picture related to ion has 34 protons what element is it
How To Teach Finding Protons Neutrons And Electrons In An Element
https://images.squarespace-cdn.com/content/v1/61a7976ff13b3127ab967596/3011a712-f1ae-453c-afd2-f7a29d6b9f8d/2.png

Protons Neutrons And Subatomic Particles Pharmacy Gyan
https://pharmacygyan.com/wp-content/uploads/2021/09/protons.jpeg
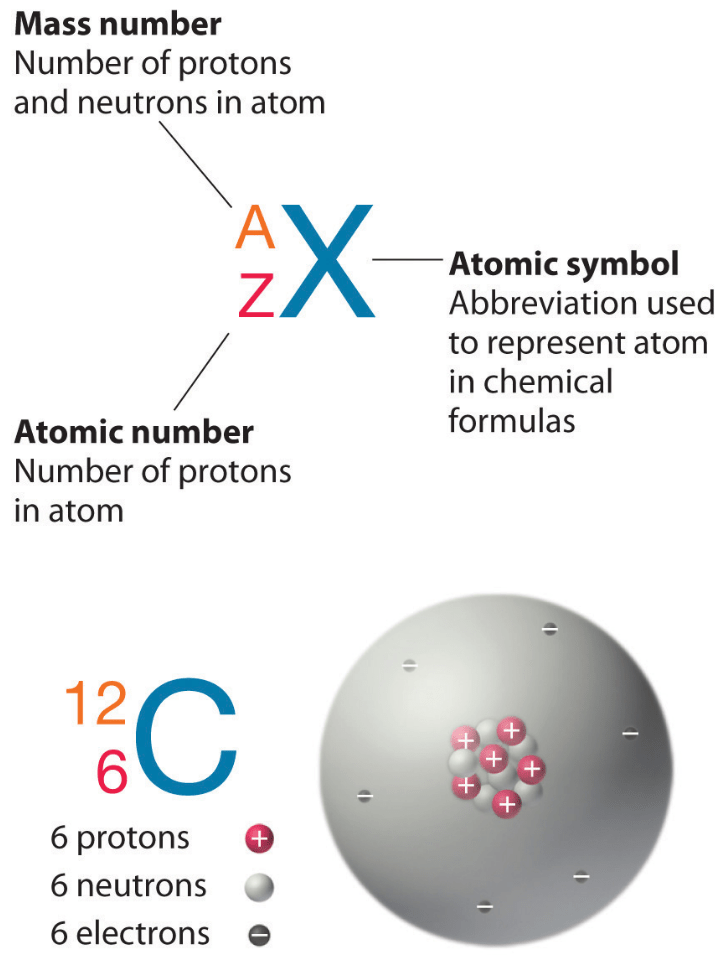
Atomic Number Mass Number Isotopes And Calculations ClassNotes ng
https://classnotes.ng/wp-content/uploads/2020/04/ATOMIC-NUMBER-MASS-NUMBER-ISOTOPES-AND-CALCULATIONS.png
Question An elemental ion has 34 protons 36 electrons and 44 neutrons What isotope is it Atomic Number and Atomic Mass An atom is made up of protons positively charged neutrons An isotope is defined by the number of protons and neutrons in its nucleus not by the number of electrons Carbon atoms have 6 protons but they can have different numbers of neutrons such as 6 7 or 8 These are called carbon 12 carbon 13 and carbon 14 respectively They are all isotopes of carbon but they have different masses and
Rhenium has 75 protons 111 neutrons and 75 electrons 76 Osmium has 76 protons 114 neutrons and 76 electrons 77 Iridium has 77 protons 115 neutrons and 77 electrons 78 Platinum has 78 protons 117 neutrons and 78 electrons 79 Gold has 79 protons 118 neutrons and 79 electrons 80 Mercury has 80 protons 121 neutrons A magnesium ion therefore has 10 electrons b Since N 3 is an anion its name is the root name of the element with the suffix ide This would make it a nitride ion Nitrogen atoms contain 7 protons in their nucleus To get a 3 charge the ion had to gain three electrons A nitride ion therefore has 10 electrons

Iron Protons Neutrons Electrons Electron Configuration
https://material-properties.org/wp-content/uploads/2020/09/Iron-protons-neutrons-electrons-configuration.png
Solved An Ion From A Given Element Has 34 Protons And 36 Chegg
https://media.cheggcdn.com/study/1c1/1c1a86ef-2b80-4264-ae6a-e98d2855758f/image
ion has 34 protons what element is it - One example of an element with 34 protons is selenium Se Selenium normally has 34 electrons to balance the 34 protons However if it loses two electrons it will form a 2 ion Se2 not a 2 ion So based on the information given there is no element that corresponds to a 2 ion with 34 protons Advertisement