what is hess law Hess s Law is named after Russian Chemist and Doctor Germain Hess Hess helped formulate the early principles of thermochemistry His most famous paper which was published in 1840 included his law on thermochemistry
Hess s law rule proposed by Germain Henri Hess stating that the heat absorbed or evolved or the change in enthalpy in any chemical reaction is a fixed quantity and is independent of the path of the reaction or the number of steps taken to obtain the reaction Hess s Law is the most important law in this part of chemistry Most calculations follow from it It says The enthalpy change accompanying a chemical change is independent of the route by which the chemical change occurs
what is hess law
what is hess law
http://www.sliderbase.com/images/referats/112b/(2).PNG
Hess s Law Presentation Chemistry
https://www.sliderbase.com/images/referats/112b/(3).PNG

PPT Hess s Law PowerPoint Presentation Free Download ID 6193634
https://image3.slideserve.com/6193634/hess-s-law-n.jpg
Hess s law states that the total enthalpy change of a reaction equals the sum of all the enthalpy changes occurring in each step of the reaction In other words net enthalpy is independent of the number of steps the reaction takes to complete Hess s law is valid so it is possible to divide a chemical reaction into different steps and calculate the total energy of the reaction using the standard enthalpies of formation Empirical data typically obtained through calorimetry
What is Hess s Law Russian Chemist and Physicist Germain Hess developed the concepts of thermochemistry and physical chemistry He introduced the concept known as Hess s Law of Constant Heat of Summation or Hess s Law for short Hess s law has to do with net enthalpy in a reaction or set of thermodynamic processes The purpose of Hess s law is to measure the enthalpies of neutralization for several acid base reactions then use that information and Hess s law to determine the reaction enthalpies for two salts in aqueous solution
More picture related to what is hess law
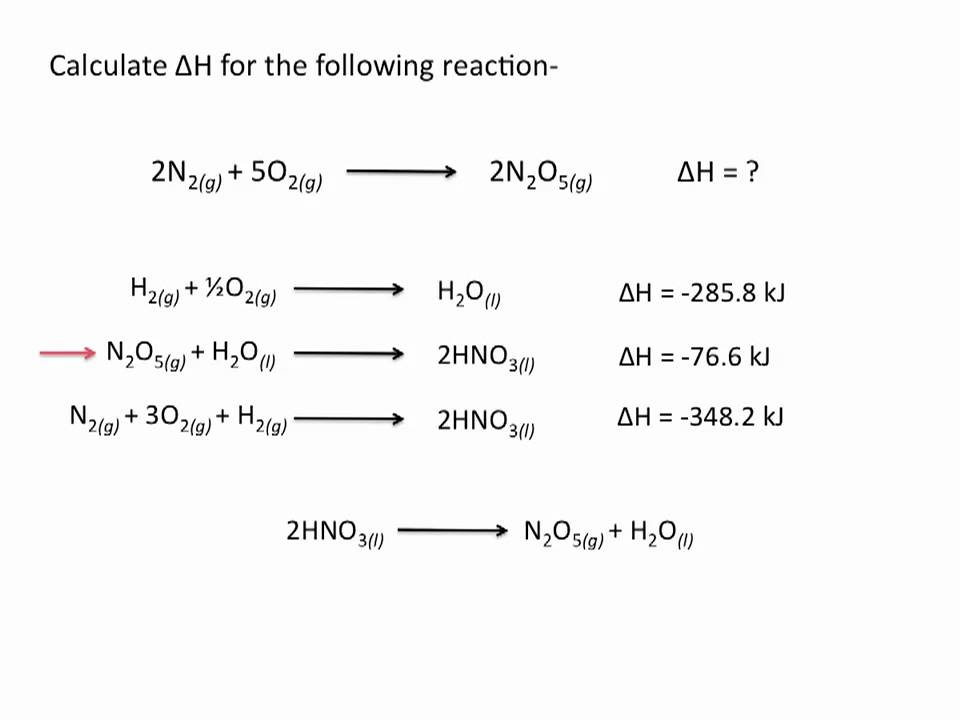
Hess s Law Chemistry Tutorial YouTube
http://i.ytimg.com/vi/iETCSFit-zA/maxresdefault.jpg
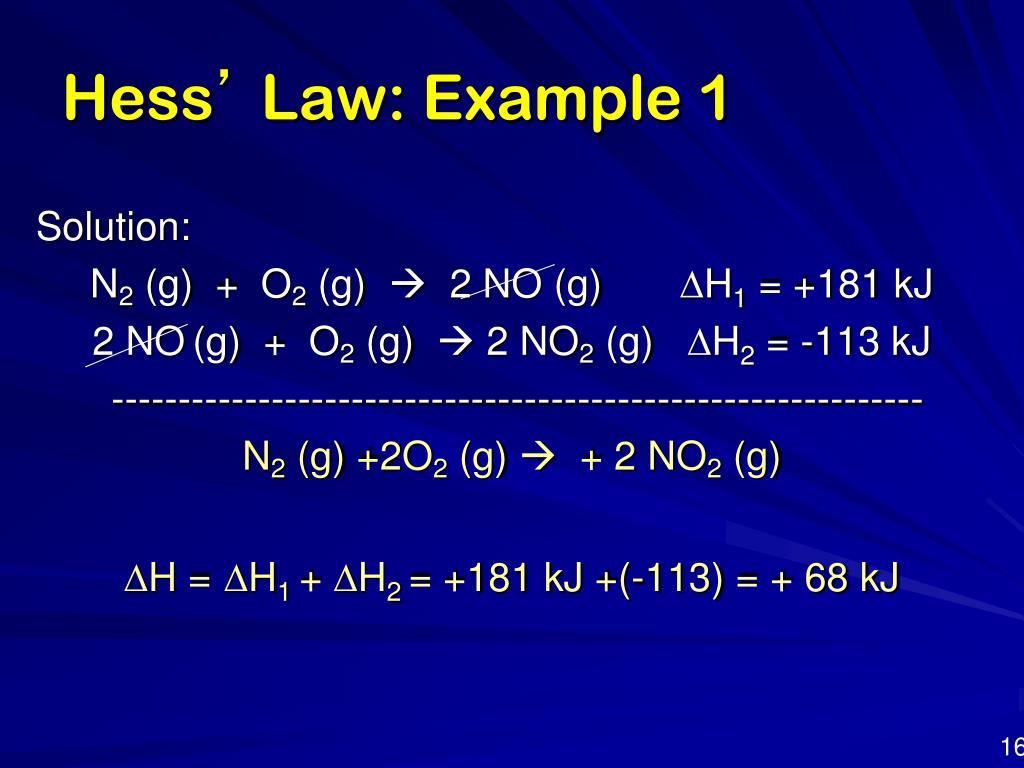
PPT Hess S Law Energetics PowerPoint Presentation Free Download
https://image3.slideserve.com/5573897/hess-law-example-11-l.jpg
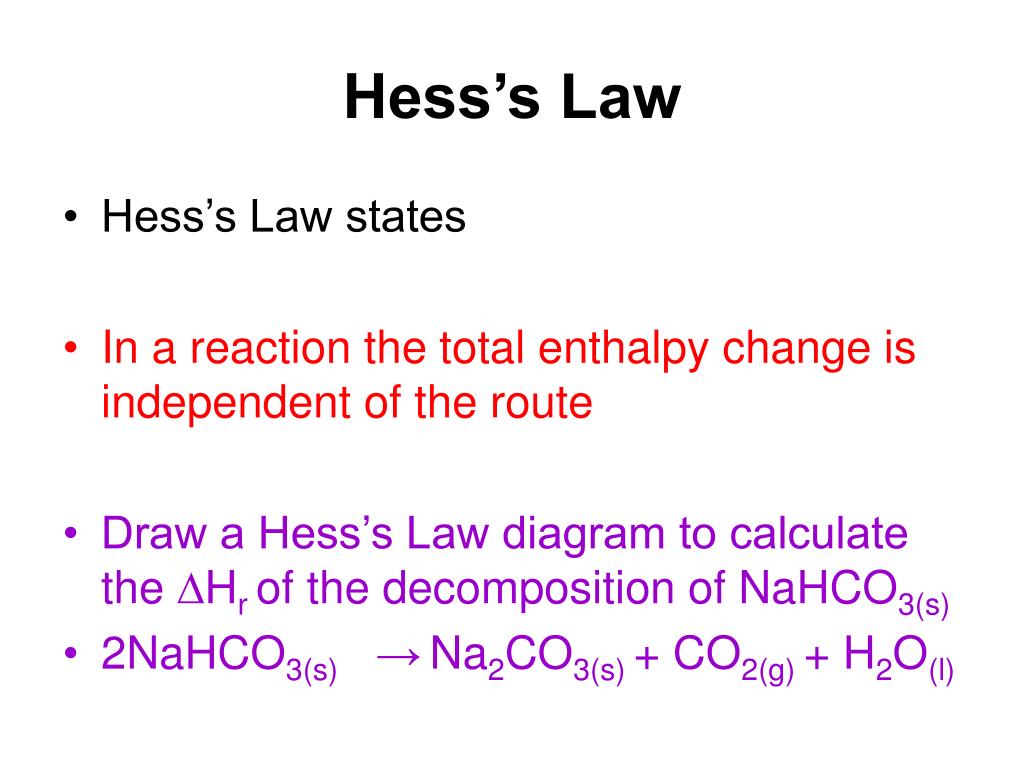
PPT Hess s Law PowerPoint Presentation Free Download ID 6193627
https://image3.slideserve.com/6193627/hess-s-law3-l.jpg
Hess law states that the change of enthalpy in a chemical reaction is the same regardless of whether the reaction takes place in one step or several steps provided the initial and final states of the reactants and products are the same This type of calculation usually involves the use of Hess s law which states If a process can be written as the sum of several stepwise processes the enthalpy change of the total process equals the sum of the enthalpy changes of the various steps
[desc-10] [desc-11]
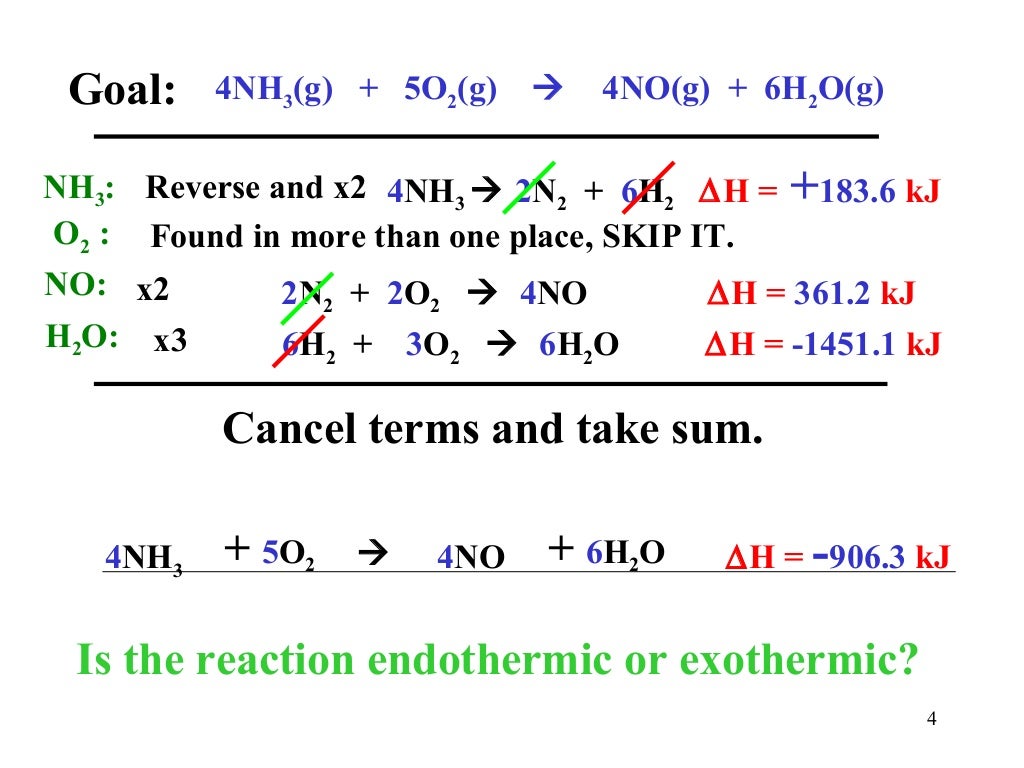
Hess s Law
https://image.slidesharecdn.com/hessslaw-140506102234-phpapp02/95/slide-4-1024.jpg

Hess s Law Thermodynamics AP Chemistry Khan Academy YouTube
https://i.ytimg.com/vi/QU3zVec5I_M/maxresdefault.jpg
what is hess law - What is Hess s Law Russian Chemist and Physicist Germain Hess developed the concepts of thermochemistry and physical chemistry He introduced the concept known as Hess s Law of Constant Heat of Summation or Hess s Law for short Hess s law has to do with net enthalpy in a reaction or set of thermodynamic processes
.PNG)
.PNG)