molar vs mol litre How many Molar make 1 Mole per liter Measurement calculator that can be used to convert mol l to M Mole per liter to Molar among others Molar concentration
How many Mole per liter make 1 Molar Measurement calculator that can be used to convert M to mol l Molar to Mole per liter among others Molar concentration This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating grams per ml to moles You can also calculate the mass of a substance needed to
molar vs mol litre

molar vs mol litre
https://i.pinimg.com/originals/2b/cb/32/2bcb32c921ab9a10a42b25ebe8c5e121.jpg
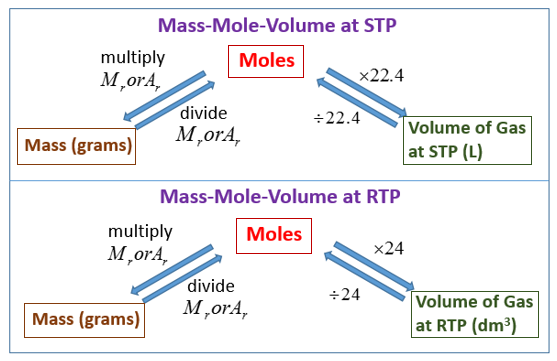
Molar Volume And Avogadro s Law video Lessons Examples And Solutions
https://www.onlinemathlearning.com/image-files/mass-mole-volume.png
, Molality(m), Molarity (M), Mole fraction (X), Percentage solution %25 , parts per million (ppm).jpg)
Normality Molality Molarity Mole Fraction Percentage Solution
https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjVG9acjdV0NhW_Ptdw7s3LlhJVDEZtREJlKBDMAJ7xtzGTEXhj2ddHRm2gldk-Fd1ORXdWK1hSbXCi7f-WPN94jbzYas9LRSEksgcR5V1OGKQl03wE1S5Cauldv4AkA95VTXfgcVMaVP1ouQ7fVkVUPEVD_wbfBoHHe4op695coYgTiklmGcD2AvE/w1600/Normality (N), Molality(m), Molarity (M), Mole fraction (X), Percentage solution %25 , parts per million (ppm).jpg
Molarity is expressed in moles per liter mol L or M while the mole is expressed in moles mol Molarity provides a concentration value whereas the mole represents a specific quantity of a The unit of molarity M or mol L simply tells you how many moles of solute are dissolved in one liter of solution For example a 1 M 1 mol L solution contains 1 mole of solute in every liter
The most common unit of concentration is molarity which is also the most useful for calculations involving the stoichiometry of reactions in solution The molarity M is defined The symbol for molarity is text M or moles liter Chemists also use square brackets to indicate a reference to the molarity of a substance For example the expression left ce Ag right refers to the molarity of the silver ion in
More picture related to molar vs mol litre
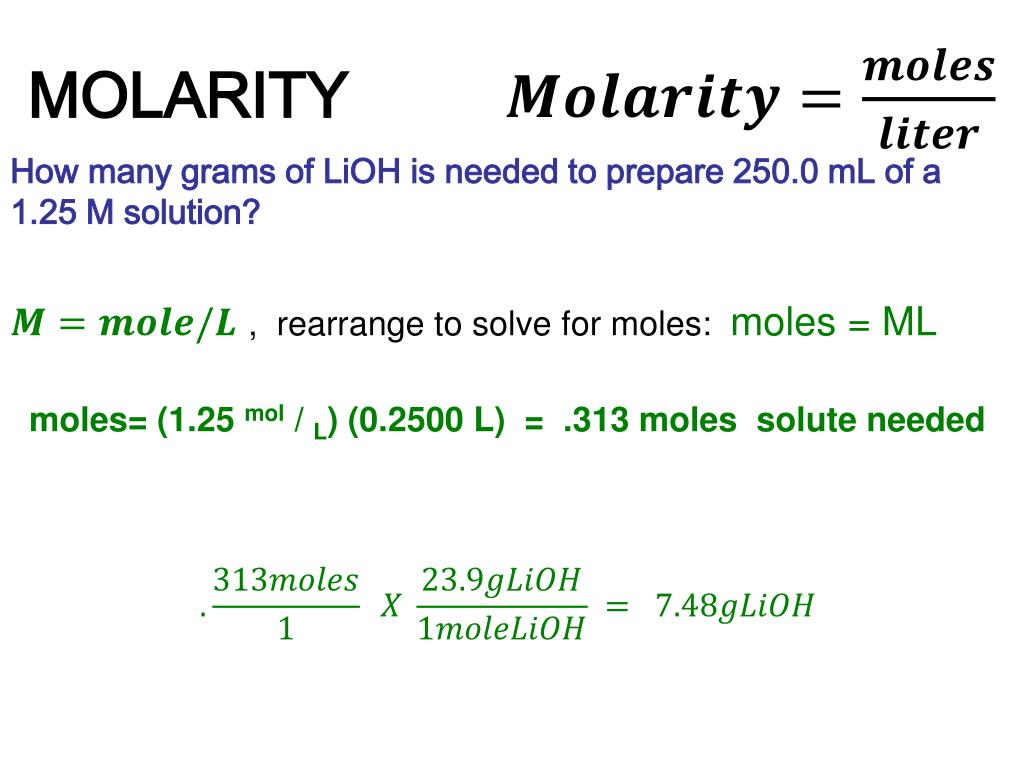
PPT MOLARITY A Measurement Of The Concentration Of A Solution
https://image.slideserve.com/1459925/slide2-l.jpg
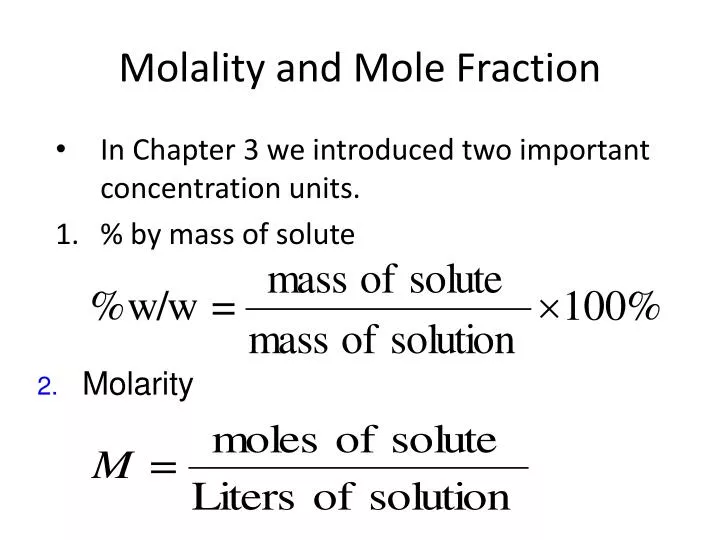
PPT Molality And Mole Fraction PowerPoint Presentation Free Download
https://image3.slideserve.com/6594493/molality-and-mole-fraction-n.jpg
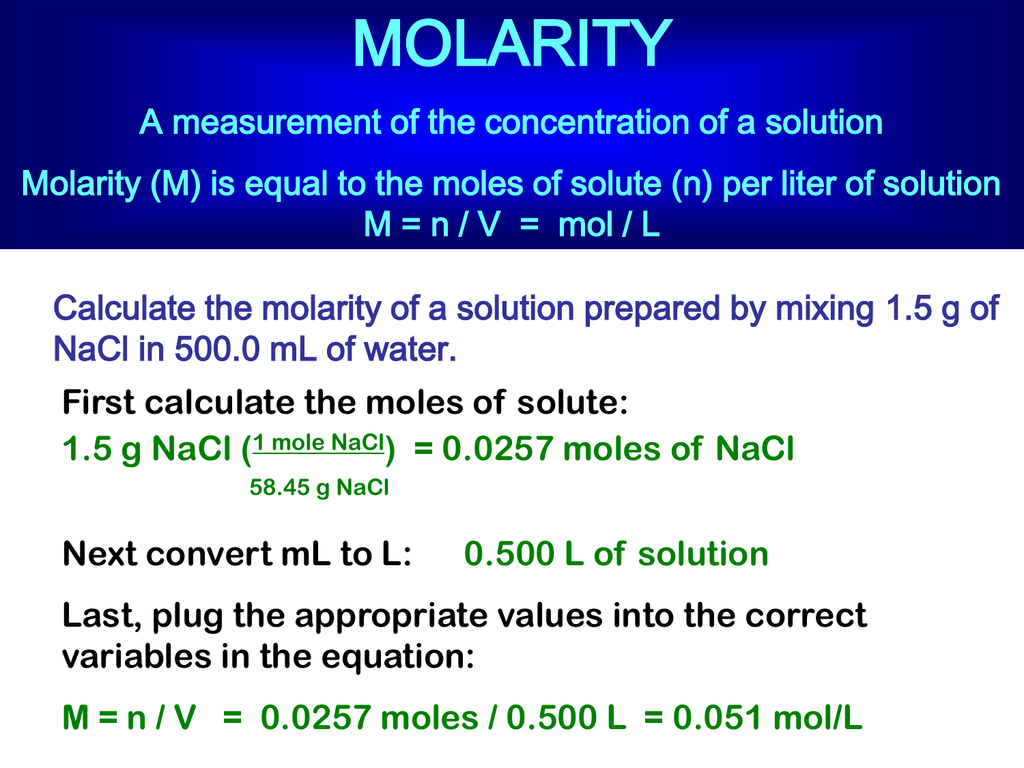
24 Molarity
https://s2.studylib.net/store/data/010171790_1-51f7fb90093cafc31a830c65daa31b6a.png
Molarity and molality are both measures of the concentration of a chemical solution Molarity is the ratio of moles to volume of the solution mol L while molality is the ratio of moles to the mass of the solvent mol kg Most of the time it doesn t matter which unit of concentration you use Molarity is the concentration of a solution in terms of the number of moles of the solute in 1 dm 3 1 liter of the solution A mole is the quantity of anything that has the same number of particles as 12 g of carbon 12 This equates to
Calculator and formulas for conversion between different units of concentration Molarity molality mole fraction weight percent of solute and grams of solute per liter of solution Calculate Mass Required for Molar Solution The molarity calculator calculates the mass of compound required to achieve a specific molar concentration and volume To dilute a solution

CHEMISTRY 101 The Mole And Molar Mass YouTube
https://i.ytimg.com/vi/VgJvX2KinTE/maxresdefault.jpg
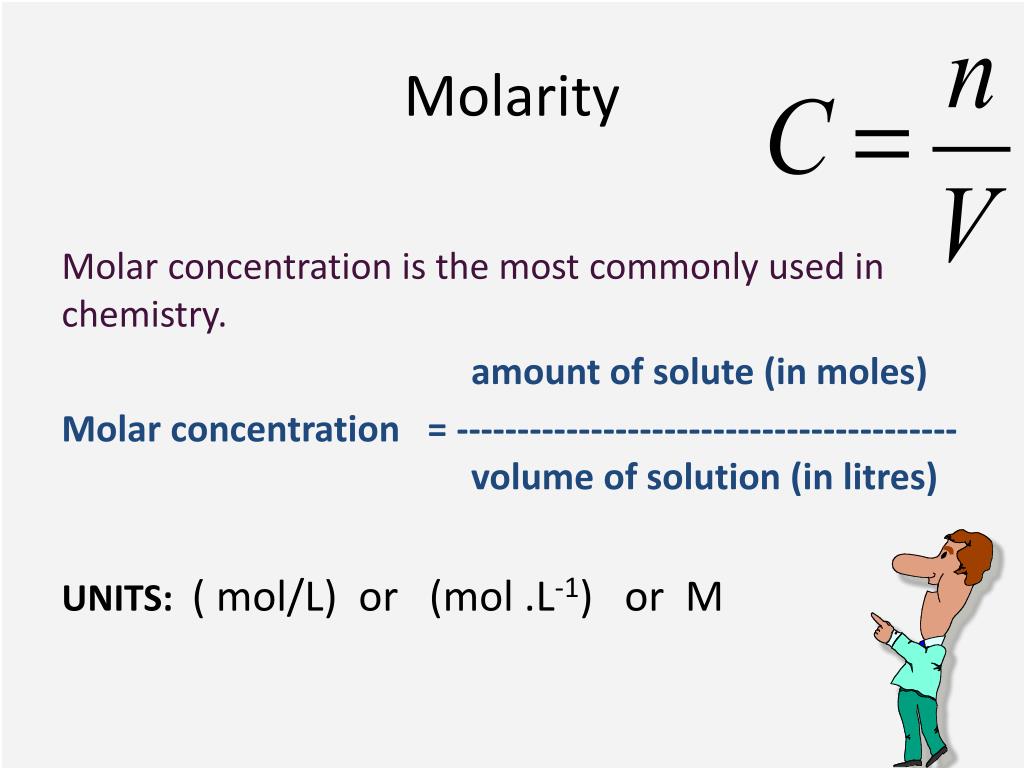
PPT Concentration PowerPoint Presentation Free Download ID 3874312
https://image2.slideserve.com/3874312/molarity-l.jpg
molar vs mol litre - The symbol for molarity is text M or moles liter Chemists also use square brackets to indicate a reference to the molarity of a substance For example the expression left ce Ag right refers to the molarity of the silver ion in