in what units is molar mass typically expressed The molar mass of a substance is defined as the mass of 1 mol of that substance expressed in grams per mole and is equal to the mass of 6 022 10 23 atoms molecules or formula units of that substance
The units of molar mass follow its definition grams per mole Mathematically the defining equation of molar mass is Molar mass mass mole g mol The definition of atomic In chemistry the molar mass M sometimes called molecular weight or formula weight but see related quantities for usage of a chemical compound is defined as the ratio between the mass and the amount of substance measured in moles of any sample of the compound The molar mass is a bulk not molecular property of a substance The molar mass is an average of many instances of th
in what units is molar mass typically expressed

in what units is molar mass typically expressed
https://i.ytimg.com/vi/ZNtQa981Bqk/maxresdefault.jpg

5 1b Calculating And Using The Molar Mass Of Elements YouTube
https://i.ytimg.com/vi/gizAUk4p3TA/maxresdefault.jpg

Molar Mass Definition Formula with Examples Concepts
https://d1avenlh0i1xmr.cloudfront.net/7539cbe8-cc56-441a-9a89-52af40792205/molar-mass-teachoo-01.jpg
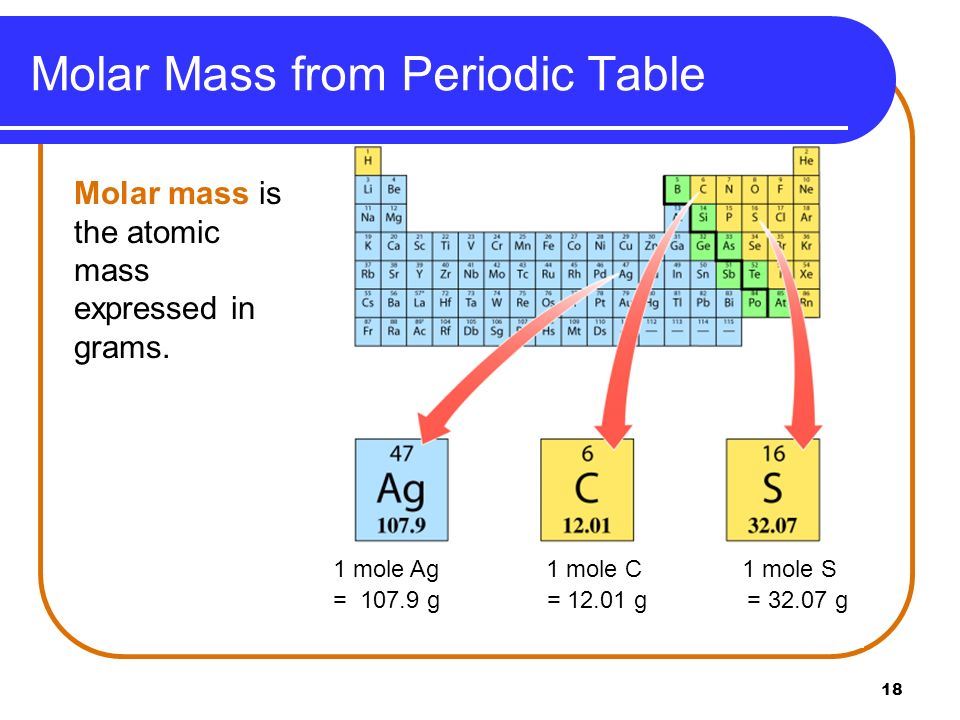
As the reference says the Standard International units for molar mass is kilograms per mole kg mol but for historical reasons molar mass is almost always expressed in grams As with atomic mass unit based masses to obtain the mass of 1 mol of a substance we simply sum the masses of the individual atoms in the formula of that substance
The base SI unit for mass is the kilogram 1 but for both practical and historical reasons molar masses are almost always quoted in grams per mole g mol or g mol 1 especially in chemistry The molar mass of a substance is defined as the mass of 1 mol of that substance expressed in grams per mole and is equal to the mass of 6 022 10 23 atoms molecules or formula units of that substance
More picture related to in what units is molar mass typically expressed

Prelab Questions Apparent Molar Mass Of Air 1 What Is A Molar Mass
https://img.homeworklib.com/questions/396895f0-c5fe-11ea-937d-d52d7652fb23.png?x-oss-process=image/resize,w_560
How To Calculate Density From Molecular Weight Haiper
https://image.slideserve.com/1459473/calculating-molar-mass1-l.jpg
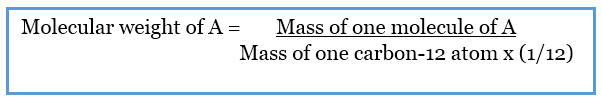
Difference Between Molar Mass And Molecular Weight Definition
https://pediaa.com/wp-content/uploads/2017/06/Difference-Between-Molar-Mass-and-Molecular-Weight-3.png
Units Molecular mass is expressed in atomic mass units amu or Daltons Da These units represent a relative scale of mass based on the carbon 12 isotope as a reference Molecular First molecular mass is either unitless or else reported in daltons Da or atomic mass units amu or u On the other hand the unit for molar mass is grams per mole g mol or kilograms per mole kg mol Second molecular
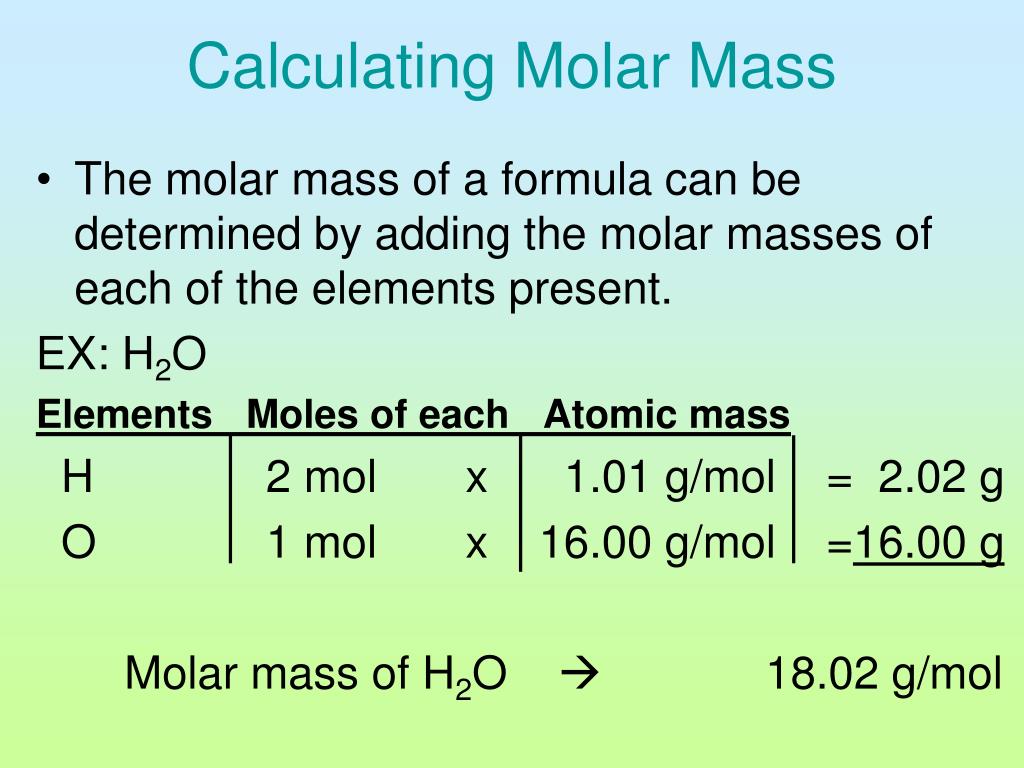
The molar mass is defined as the mass of 1 mol which is in the SI measured in g mol Example The molar mass of water H 2O MM 18 016 g mol The mass of one mole of a substance in grams is known as its molar mass Molar mass is measured in grams per mole or g mol In other words the molar mass is the sum of

Molarity Molality Volume Mass Percent Mole Fraction Density
https://i.ytimg.com/vi/NL8quYmMOMs/maxresdefault.jpg
Atomic Mass And Molecular Mass Definition Difference Mass Spectrometry
https://cdn1.byjus.com/chemistry/wp-content/uploads/2016/01/slide_18.jpg
in what units is molar mass typically expressed - The molar mass of a substance is defined as the mass of 1 mol of that substance expressed in grams per mole and is equal to the mass of 6 022 10 23 atoms molecules or formula units of that substance