human factors medical device conference 4 th Annual European Medical Device Human Factors and Usability Engineering Conference TBC Europe We Unleash Innovation Explore AI Human Factors IFU Optimization Patient Centric Design Feedback Loops and Post Market Surveillance to Transform Healthcare Key Topics for 2024 Human factors AI in the product
Medical Device Human Factors Conference November 8 9 2023 Arlington VA Positioning HFE as a Strategic Partner throughout the Full Product Development Lifecycle to Meet Diverse Patient Provider Needs within an Ever Evolving Increasingly Digitized Healthcare Setting Day 1 Agenda Day 2 Agenda Sponsors This educational event is your opportunity to learn insights on the latest science and best practices understand innovations in the safety of healthcare providers and patients sharpen the focus of HF E initiatives and improve your regulatory approaches
human factors medical device conference

human factors medical device conference
https://assets-global.website-files.com/5ec3efa8b380ded8f9609b83/6079afbc022347d010d53ac2_Domain of Human Factors.png

Benefits Of Human Factors Engineering For Medical Devices
https://www.mindflowdesign.com/wp-content/uploads/2019/11/img_5968-r1-1024x768.jpg
Human Factors Engineering For Medical Devices EMMA International
https://emmainternational.com/wp-content/uploads/2021/01/HFE-Med-Devices_Blog-01-768x512.jpg
Annual Medical Device Human Factors and Usability Engineering Conference is designed to bring together luminaries professionals and researchers from various disciplines to explore the critical role of human factors and usability engineering in ensuring medical devices safety effectiveness and user satisfaction 4 th Annual European Medical Device Human Factors and Usability Engineering Conference
Based on papers presented at the AHFE 2021 Conference on Human Factors and Ergonomics in Healthcare and Medical Devices held virtually on 25 29 July 2021 from USA the book offers a timely reference guide to both researchers and healthcare professionals involved in the design of medical systems and managing US Annual Medical Device Human Factors and Usability Engineering Conference TBC Hilton Back Bay Boston USA
More picture related to human factors medical device conference
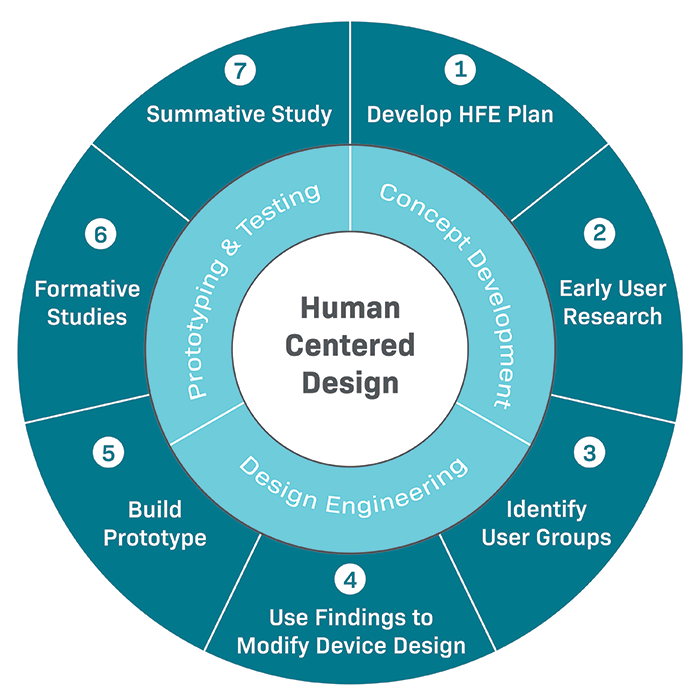
The Importance Of Human Factors For Medical Devices Gilero
https://www.gilero.com/wp-content/uploads/2020/04/Human-Factors-Infographic-1.png

How To Classify A Class III Medical Device
https://sterlingmedicaldevices.com/app/uploads/2021/04/Product-development-scaled.jpg

Three Takeaways From The Human Factors Engineering Usability Studies
https://sterlingmedicaldevices.com/app/uploads/2021/01/human_factors.jpg
Discover unmet user needs in sessions with regulatory authorities and medical device peers Speakers will share evolving FDA requirements and practical and effective testing roadmaps to ensure needs are translated into design concepts AHFE 2021 25 29 July Advances in Neuroergonomics and Cognitive Engineering 60 Papers 1 Volume Advances in Industrial Design 138 Papers 1 Volume Advances in Ergonomics in Design
3 rd Annual European Medical Device Human Factors and Usability Engineering Conference 15th 16th May 2024 Steigenberger Airport Hotel Berlin Germany Conference Registration Let s get you registered To initiate the registration process please complete the form The US Annual Medical Device Human Factors and Usability Engineering Conference is designed to bring together experts professionals and researchers from various disciplines to explore the critical role that human factors and usability engineering play in ensuring medical devices safety effectiveness and user satisfaction

Applying Human Factors And Usability Engineering To Medical Devices
https://sterlingmedicaldevices.com/app/uploads/2021/11/applying-human-factors-and-usability-engineering-to-medical-1.png

An Introduction To Human Factors Engineering In Medical Device Design
https://arrotek.com/wp-content/uploads/2021/05/An-Introduction-to-Human-Factors-Engineering-in-Medical-Device-Design.jpg
human factors medical device conference - 4 th Annual European Medical Device Human Factors and Usability Engineering Conference