how to find inflection point on titration curve The simplest method for finding the end point is to locate the titration curve s inflection point which is shown by the arrow This is also the least accurate method particularly if the titration curve has a shallow slope at the equivalence point See Chapter 11 for more details about pH electrodes Figure 9 2 9
The equivalence point of a titration Sorting out some confusing terms Titration curves for strong acid v strong base The graph is showing two end points one at a pH of 8 3 little more than a point of inflexion and a second at about pH 3 7 The reaction is obviously happening in two distinct parts There is just what we call a point of inflexion at the equivalence point Lack of any steep change in pH throughout the titration renders titration of a weak base versus a weak acid difficult and not much information can be extracted from such a curve
how to find inflection point on titration curve

how to find inflection point on titration curve
https://media.nagwa.com/302142137591/en/thumbnail_l.jpeg
Endpoint Titration
https://img.brainkart.com/imagebk30/Y9Da2uJ.jpg

Question Video Finding The Inflection Points Of A Function From The
https://media.nagwa.com/456137148905/en/thumbnail_l.jpeg
1 Answer Sorted by 4 begingroup Let s suppose we want to titrate a solution containing an unknown monoprotic and weak acid We use a strong base such as ce NaOH When the number and moles of hydroxide ions is equal to the amount of hydronium ions here we have the equivalence point A titration curve is a graphical representation of the pH of a solution during a titration In a strong acid strong base titration the equivalence point is reached when the moles of acid and base are equal and the pH is 7
Titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another solution This is done by carefully measuring out a specific volume of the first solution called the titrant and adding it to the second solution called the analyte until the reaction is complete It can be calculated precisely by finding the second derivative of the titration curve and computing the points of inflection where the graph changes concavity however in most cases simple visual inspection of the curve will suffice
More picture related to how to find inflection point on titration curve

Logistic Fx Equation Explination Kesilinternational
https://media.nagwa.com/873167374575/en/thumbnail_l.jpeg

Locating A Titration s Equivalence Point Image And Video Exchange
https://asdlib.org/imageandvideoexchangeforum/files/2013/07/Figure9.11.jpg
Titration Labeled
https://image.slideserve.com/225155/a-typical-titration-curve-l.jpg
Because you have this really steep titration curve like this you could have used any of the three acid base indicators to find the equivalence point for your titration All right next let s look at the titration curve for the titration of a weak acid with a strong base In titrations involving 1 1 stoichiometry of reactants the equivalence point is the steepest point of the titration curve This is true of acid base complexometric and redox titrations as well My question is does this apply to all acid base titrations strong strong and weak strong
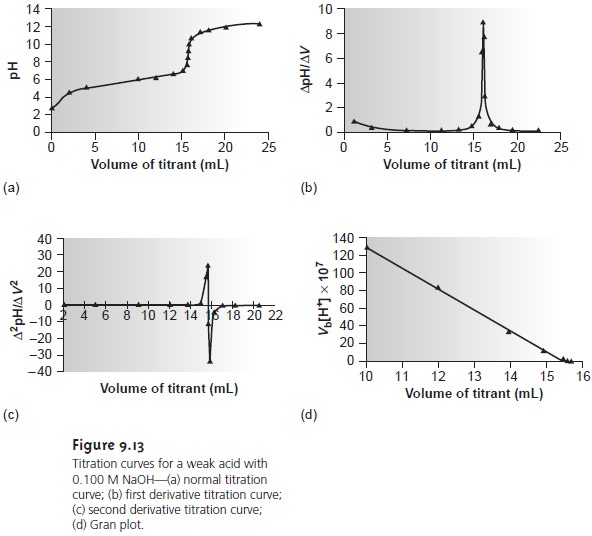
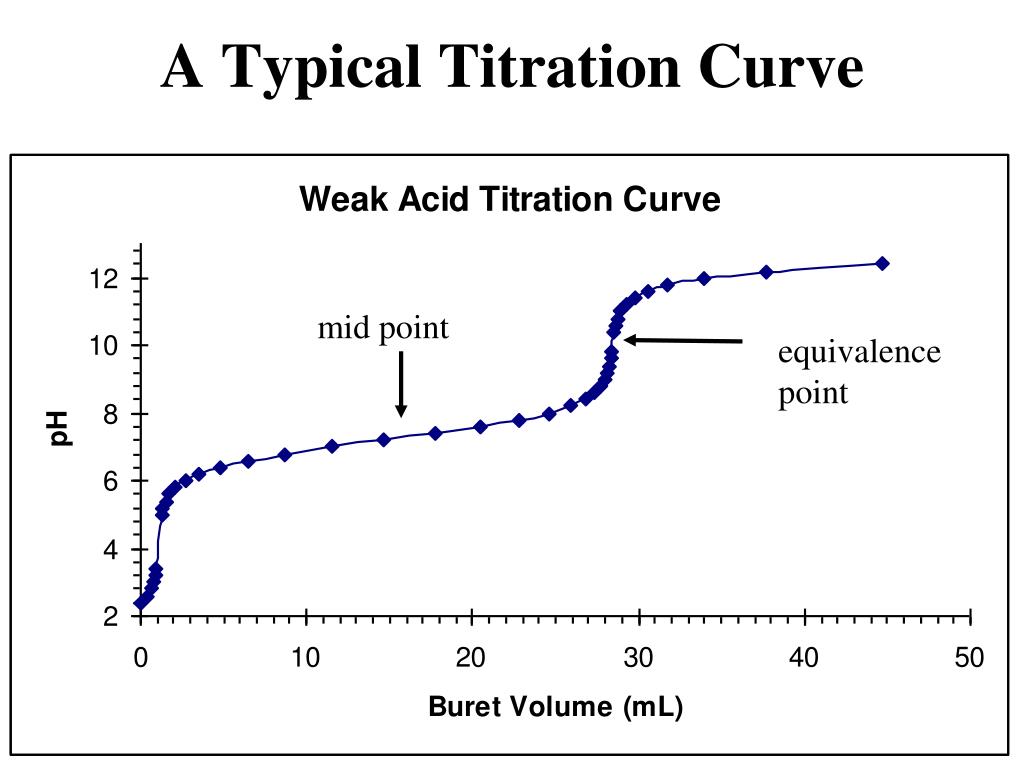
Point 1 marks the pH at the beginning of the titration before any strong acid has been added Point 2 marks the pH at 10 mL the half equivalence point Point 3 marks the pH at the equivalence point Point 4 marks the point past the equivalence point at 30 mL where excess strong acid has been added Weak Acid Strong Base Titration Curves Most titration curves have the same shape a plateau in the first part and a sharp rise or fall near equivalence point inflection point of the curve which is followed by a second flat part The most important part of the curve is the one where the changes are the fastest close to the inflection point

What Is A Titration Curve Overview Parts Expii
https://d20khd7ddkh5ls.cloudfront.net/8503_text_eb.png

Locating A Titration s Equivalence Point Image And Video Exchange
http://community.asdlib.org/imageandvideoexchangeforum/files/2013/07/Figure9.9.jpg
how to find inflection point on titration curve - 1 Answer Sorted by 4 begingroup Let s suppose we want to titrate a solution containing an unknown monoprotic and weak acid We use a strong base such as ce NaOH When the number and moles of hydroxide ions is equal to the amount of hydronium ions here we have the equivalence point