gram molecular mass Since 2019 a mole of any substance has been redefined in the SI as the amount of that substance containing an exactly defined number of particles 6 022 140 76 1023 The molar mass of a compound in g mol thus is equal to the mass of this number of molecules of the compound in grams
The bridge between the particulate and the macroscopic levels is molar mass the mass in grams of one mole of a substance The units of molar mass follow its definition grams per mole Mathematically the defining equation of molar mass The molar mass is defined as the mass of a given substance divided by the amount of a substance is expressed in grams per mol g mol That makes the molar mass an average of many particles or molecules and the molecular mass the
gram molecular mass
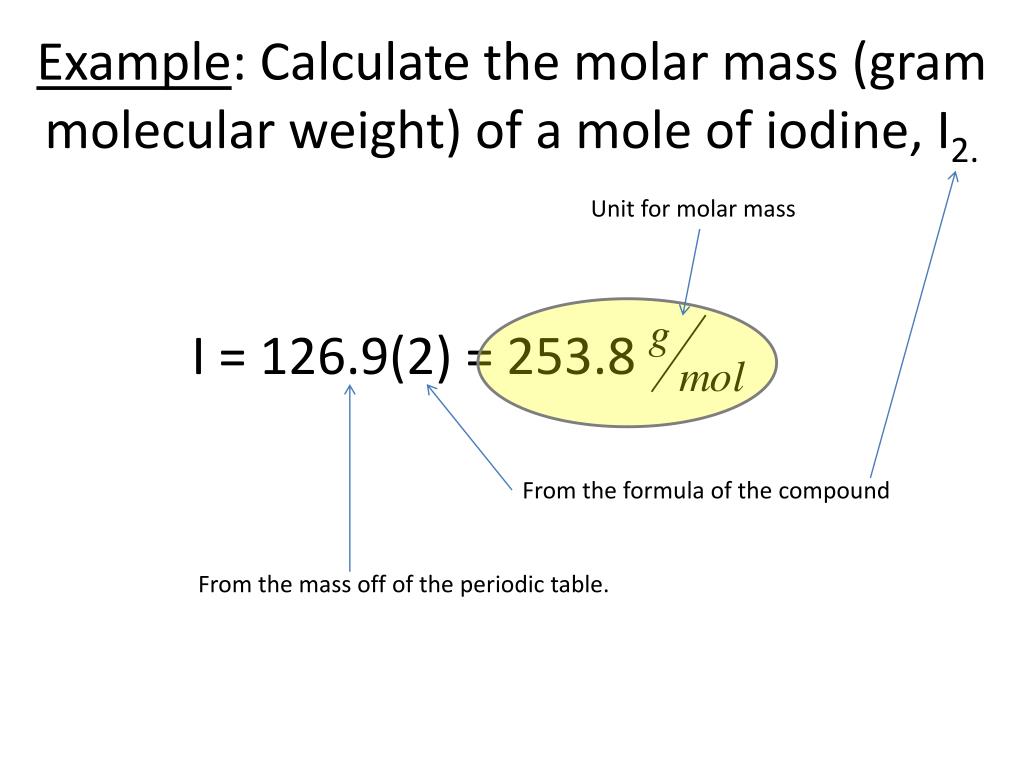
gram molecular mass
https://image1.slideserve.com/2427770/example-calculate-the-molar-mass-gram-molecular-weight-of-a-mole-of-iodine-i-2-l.jpg

Gram Atomic Mass Gram Molecular Mass Gram Formula Mass Gram Atom
https://i.ytimg.com/vi/23w9nk0BZro/maxresdefault.jpg
Atoms And Molecules Class 9 Notes Science MyCBSEguide
https://media-mycbseguide.s3.amazonaws.com/images/static/revise/09/science/09_sc_ch03_09.jpg
Gram Molecular Weight The mass in grams of 1 mole of a molecular compound is called the gram molecular weight It is numericaly equal to the molecular weight Let s look at some examples c Copyright 1999 The simplest type of manipulation using molar mass as a conversion factor is a mole gram conversion or its reverse a gram mole conversion We also established that 1 mol of Al has a mass of 26 98 g Example PageIndex 1 Stated mathematically 1 mol Al
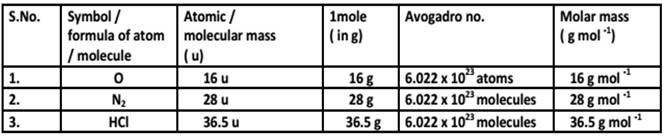
That is the molar mass of a substance is the mass in grams per mole of 6 022 10 23 atoms molecules or formula units of that substance In each case the number of grams in 1 mol is the same as the number of atomic mass units that describe the atomic mass the molecular mass or the formula mass respectively Gram atomic mass is the periodic table element s atomic weight in grams The molar mass of an element is the mass of one mole in grams The mass of one mole of an element is defined as its gram atomic mass It is calculated by taking an element s atomic weight from the periodic table and converting it to grams
More picture related to gram molecular mass
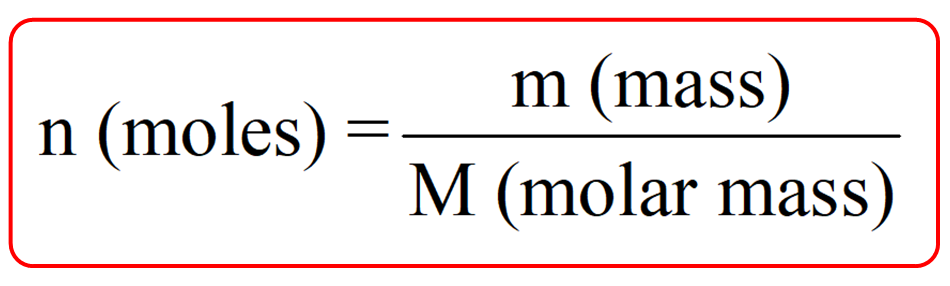
The Mole And Molar Mass Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/04/mole-mass-molar-mass-formula.png
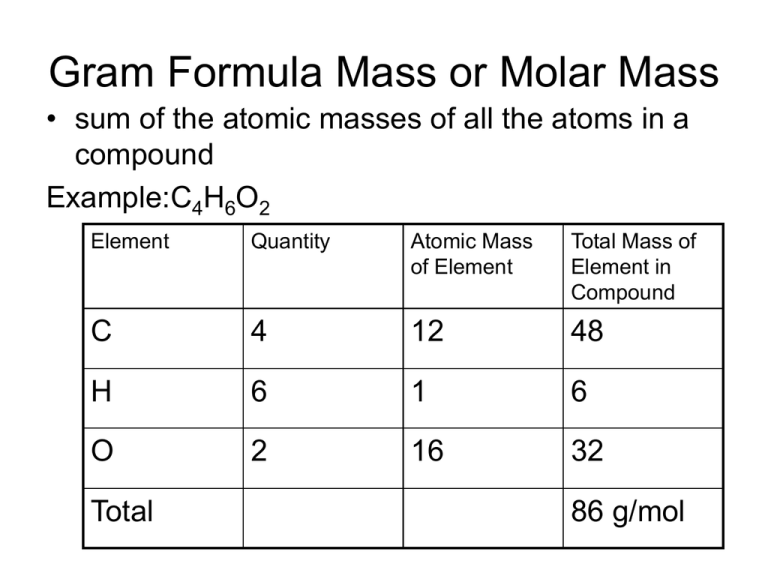
Gram Formula Mass Or Molar Mass
https://s2.studylib.net/store/data/010009192_1-509df3fbf09ffd810a4197eb8bfe1734-768x994.png

What Is The Atomic Mass Of Calcium
https://d1hj4to4g9ba46.cloudfront.net/questions/1839841_1881852_ans_54a6207737414936a0876e643fcb8941.png
Sum the masses to find the molecular mass or formula mass molecular mass 342 3 amu formula mass 164 10 amu Explanation It is called a molecular mass since C 12 H 22 O 11 is a molecular compound It is called a formula mass since Ca NO 3 2 is an ionic compound Interpretation One molecule of C 12 H 22 O 11 has a Solution Gram molecular mass It is defined as the mass of one mole molecule of an element One mole molecule contains 6 02 10 23 molecules It is represented in gram It is equal to the molar mass of a molecule Example Gram molecular mass of Oxygen O 2 Molar mass of oxygen 2 x 16 32g Suggest Corrections 17 Similar
[desc-10] [desc-11]

Difference Between Gram Atomic Mass And Gram Molecular Mass Compare
https://www.differencebetween.com/wp-content/uploads/2019/10/Difference-Between-Gram-Atomic-Mass-and-Gram-Molecular-Mass-Tabular-Form.jpg

Gram Formula Mass Guruchemist
https://2.bp.blogspot.com/-mFLbLnr1BDc/W4yjkdQ9FbI/AAAAAAAAAEY/2cL5ekHv1200nYOu5fHLSC8TnPz44qGcACEwYBhgL/s1600/Gram%252BFormula%252BMass%252BThe%252Bmass%252Bof%252Bone%252Bmole%252Bof%252Ban%252Bionic%252Bcompound..jpg
gram molecular mass - [desc-14]