g to mol 28 96 g mol This is a fun question According to table 5 1 on page 155 of Atmospheric Science An Introductory Survey By John M Wallace Peter V Hobbs table can be seen here on Google Books dry air is composed of Nitrogen 78 084 Oxygen 20 946 Argon 0 934 Carbon dioxide 0 03 If you add these up you get 78 084 20 946 0 934 0 03
Solution Set up a table for easy calculation Osmolarity 141 96 mOsmol 562 5mL 1000mL 1 L 252 mOsmol L Answer link You multiply the molarity by the number of osmoles that each solute produces An osmole Osmol is 1 mol of particles that contribute to the osmotic pressure of a solution For example NaCl dissociates completely in Where is the density m is the mass and V is the volume of the sample We can rearrange the formula to get m V mass 10 00mL 1 080 g 1mL 10 80 g n 10 80g 1 mol 102 1g 0 1058 mol MOLES FROM VOLUME OF SOLUTION Molarity is the number of moles of a substance in one litre of solution
g to mol

g to mol
https://sciencenotes.org/wp-content/uploads/2022/08/Moles-to-Grams-Conversion-1024x683.png
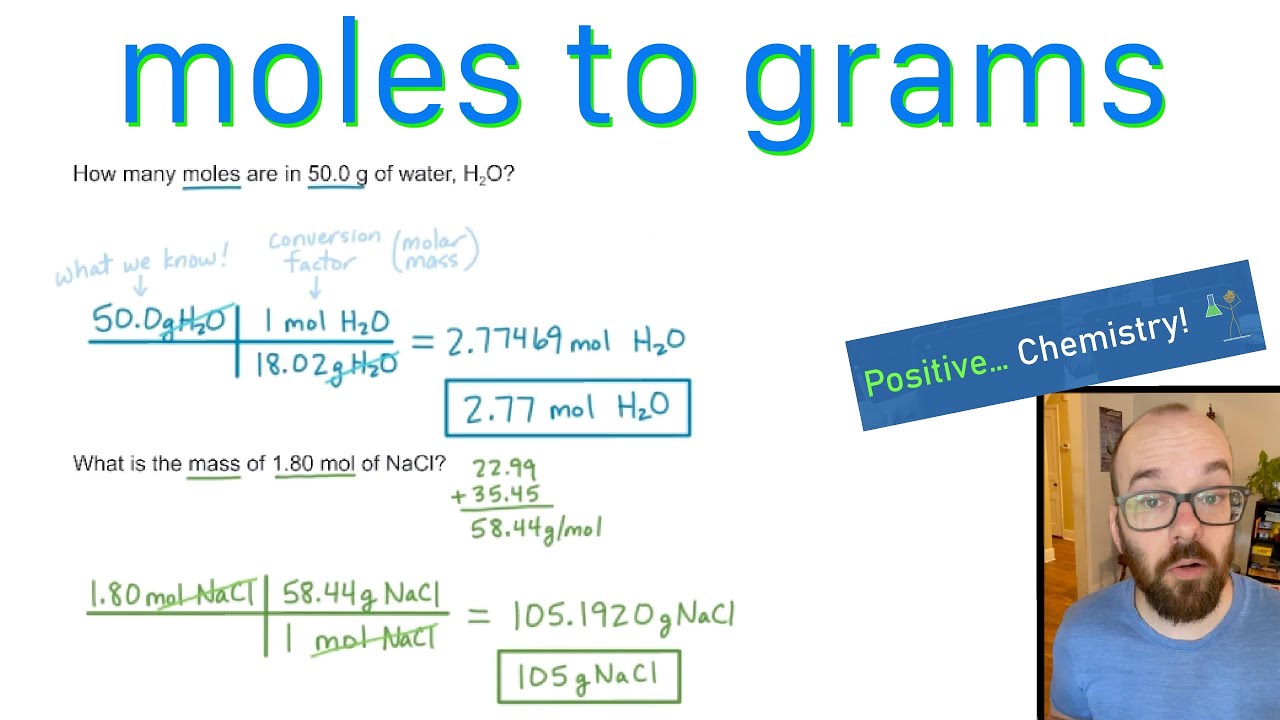
Moles To Grams How To Convert Positive Chemistry YouTube
https://i.ytimg.com/vi/Hr8jHRquYuA/maxresdefault.jpg
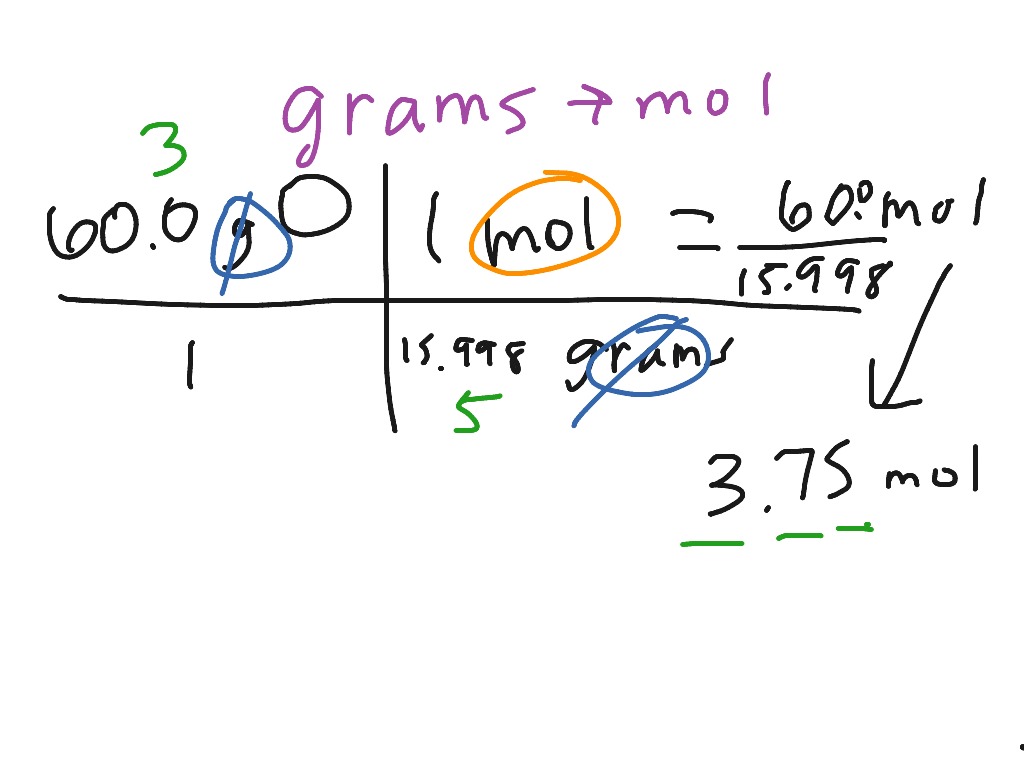
Showme Grams Free Hot Nude Porn Pic Gallery
https://showme0-9071.kxcdn.com/files/160329/pictures/thumbs/348159/last_thumb1348795592.jpg
Chloride 2 35 45 70 9 g mol approximately 63 546 70 9 134 446 Therefore the molar mass of Copper II Chloride is approximately 134 446 g mol NOTE g mol grams per mole Answer link Approximately 134 446 g mol Copper II Chloride or CuCl 2 is composed of Copper Cu and Chloride Cl 2 We can find the molar mass by adding A quick look in the periodic table and you ll see that the molar masses of sodium and chlorine respectively are Na 22 989770 g mol Cl 35 453 g mol Therefore the molar mass of sodium chloride will be M M 22 989770 g mol 35 453 g mol 58 44277 g mol In stoichiometric calculations this value is often used as 58 44 g mol
Why would you want to do so Well there are 454 g lb 1 and so were we to convert the atomic mass of say 12C we would take the quotient Sulfuric acid s molar mass is 98 08 g mol Step one Find the atomic masses in each atom units g mol Hydrogen 1 01 Sulfur 32 06 Oxygen 16 Step two Find how many of those atoms are of the formula Hydrogen 2 atoms Sulfur 1 atom Oxygen 4 atoms Step three Add all the atomic masses together Note if there are more than one of a particular atom you
More picture related to g to mol
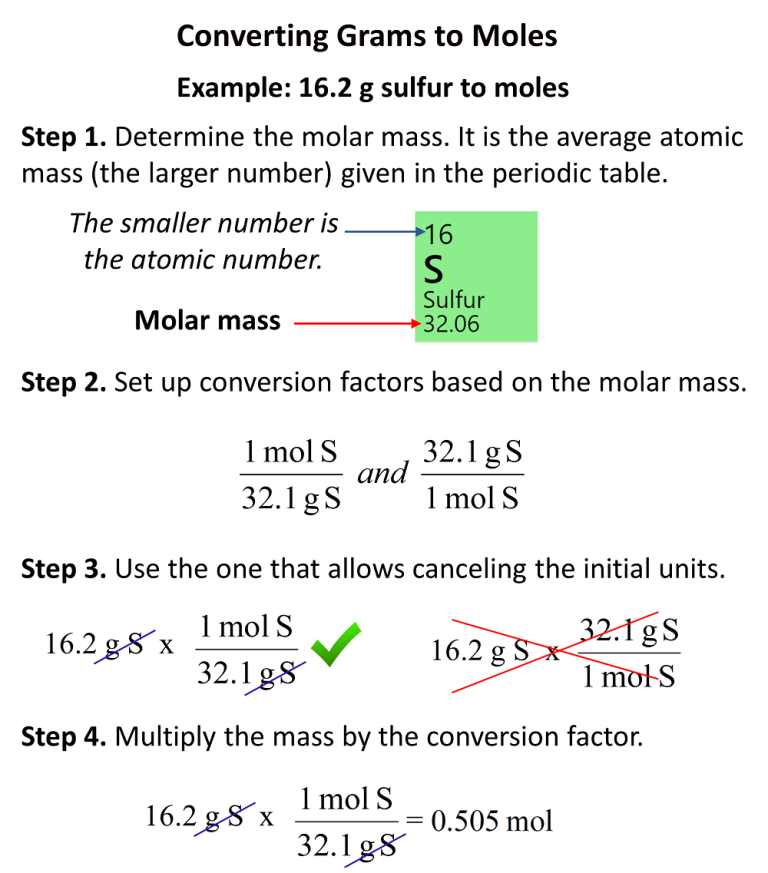
How To Convert Grams To Moles Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/12/Converting-grams-to-moles-easy-sumarry-steps-768x873.png
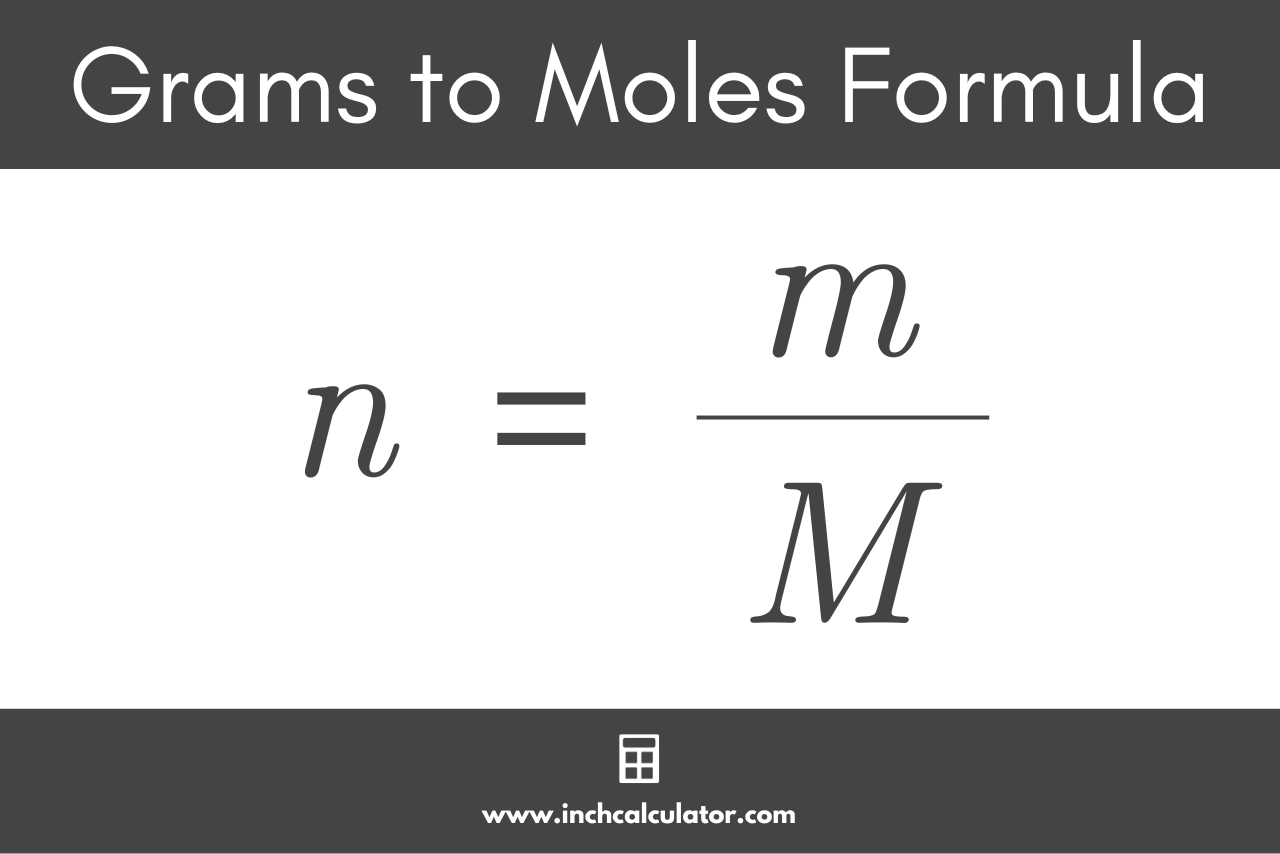
Chemistry Conversion Chart Moles To Grams
https://www.inchcalculator.com/wp-content/uploads/2023/01/grams-to-moles-formula.png
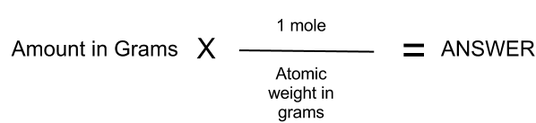
Moles Grams Conversion Chemistry Fun Facts
http://chemistryfunfacts.weebly.com/uploads/5/2/2/4/5224712/4530648.png?538
1 Answer Meave60 Dec 12 2015 The molar mass of magnesium is 24 305 g mol Explanation The molar mass of an element is its atomic weight relative atomic mass on the periodic table in g mol The atomic weight of magnesium is 24 305 therefore its molar mass is C 6 H 12 O 6 A compound s empirical formula tells you the smallest whole number ratio between the elements that make up said compound is This means that you can think about the empirical formula as being a sort of building block for the molecule Looking at the empirical formula for your compound CH 2 O you know that you need one carbon atom
[desc-10] [desc-11]
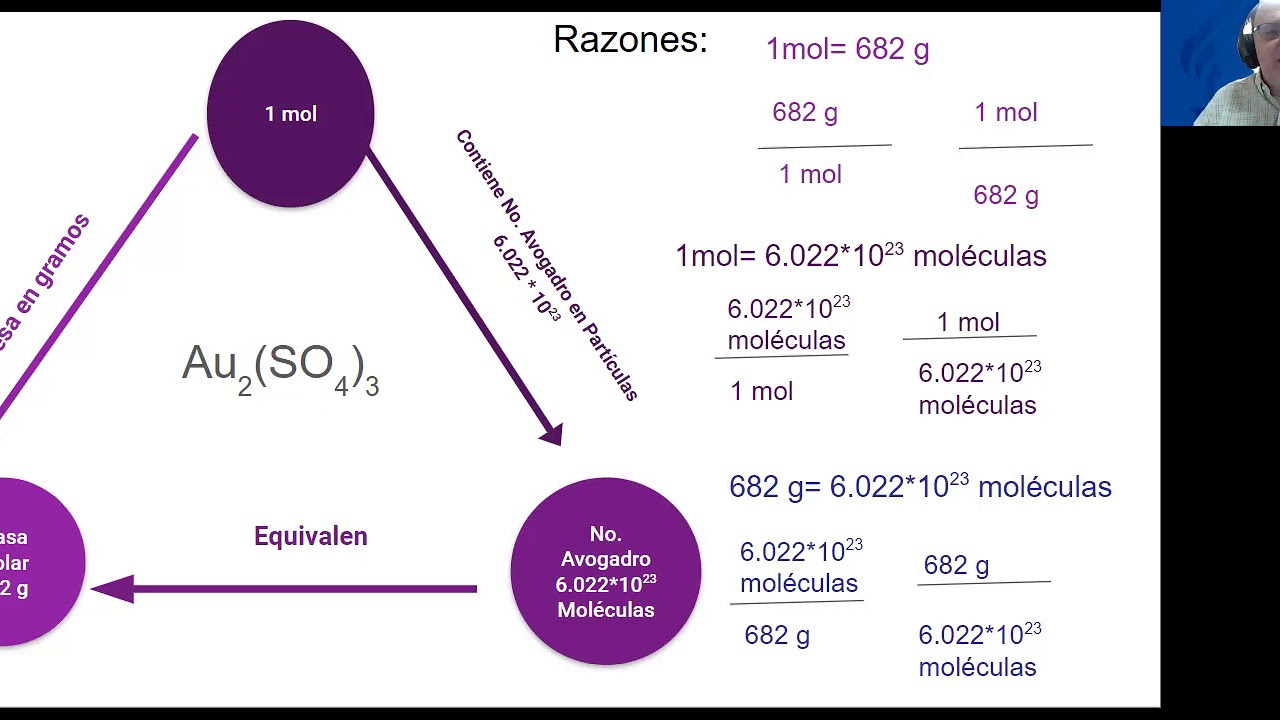
Conversiones Mol G G Mol Mol Mol culas YouTube
https://i.ytimg.com/vi/AbICqKtTML4/maxresdefault.jpg
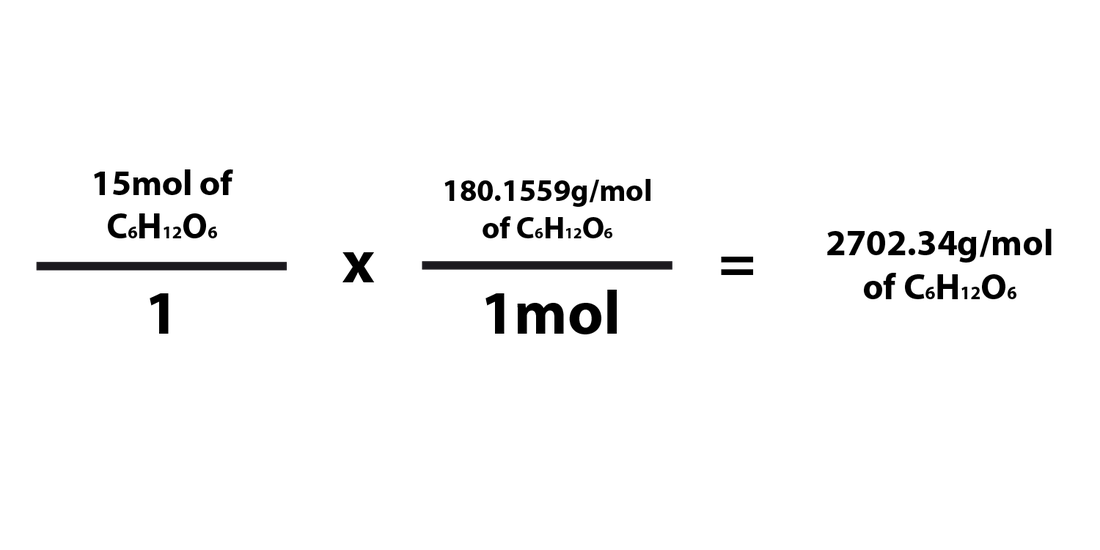
Converting Grams Moles The Mole And Its Applications
http://chemistrymoleapplications.weebly.com/uploads/7/6/6/3/76634243/7075893_orig.png
g to mol - Sulfuric acid s molar mass is 98 08 g mol Step one Find the atomic masses in each atom units g mol Hydrogen 1 01 Sulfur 32 06 Oxygen 16 Step two Find how many of those atoms are of the formula Hydrogen 2 atoms Sulfur 1 atom Oxygen 4 atoms Step three Add all the atomic masses together Note if there are more than one of a particular atom you