1 molar is equal to Molar mass of a substance is the mass in grams of one mole of the compound In a substance the amount of entities present e g atoms molecules ions is defined as a mole A mole of any substance is 6 022 1023 molecules
The molar mass of a substance is defined as the mass of 1 mol of that substance expressed in grams per mole and is equal to the mass of 6 022 10 23 atoms molecules or formula units of that substance Formula of Molar Concentration The molar concentration formula is given by Molar Concentration Amount in Moles Volume of Solution Solved Examples Example 1 Determine the molar concentration of NaOH for the reaction between HCl and NaOH Solution The balanced chemical equation can be framed as HCl NaOH NaCl H 2
1 molar is equal to
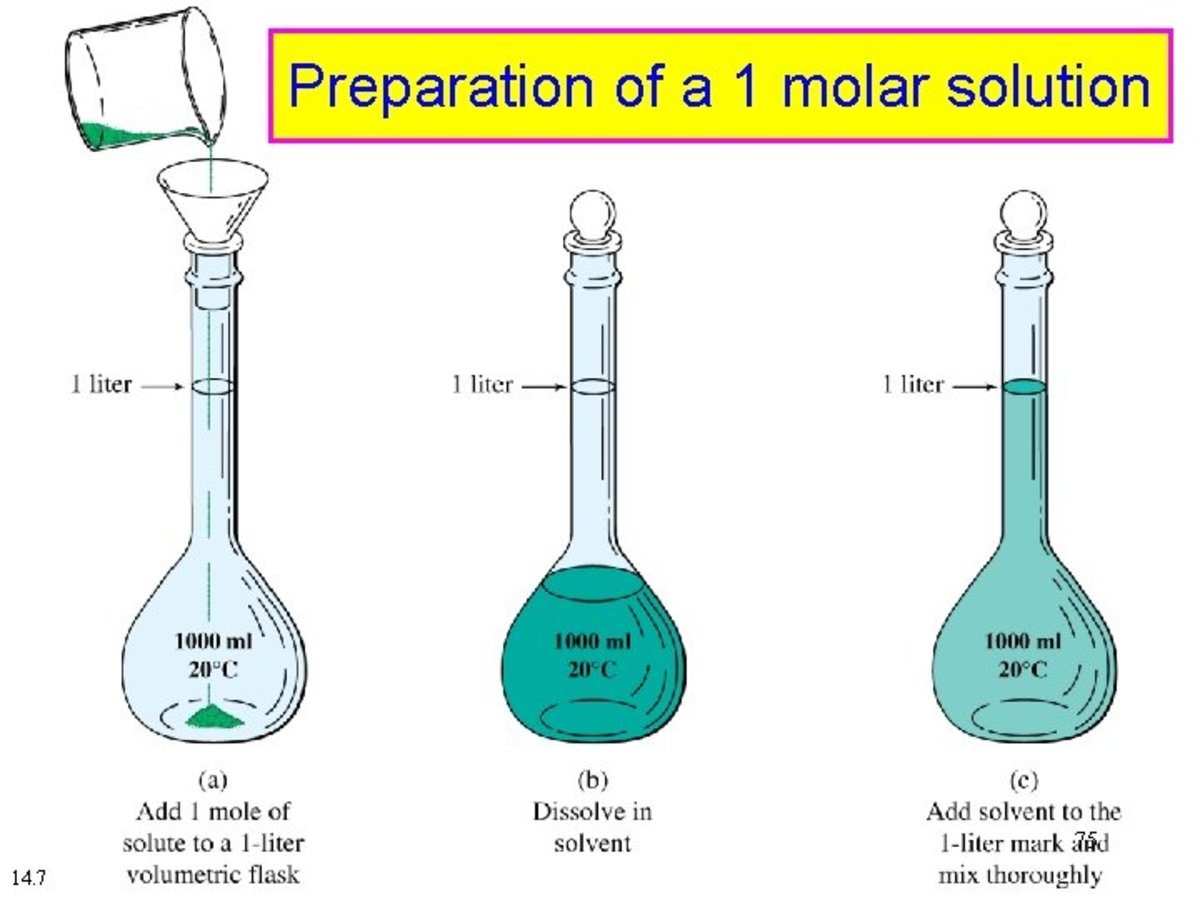
1 molar is equal to
https://images.saymedia-content.com/.image/t_share/MTg2OTcyMzI5NDU1MTk5NzM2/molarity.jpg

MOLAR AND EQUIVALENT CONDUCTIVITY PART 02 YouTube
https://i.ytimg.com/vi/c0qtIQn6cME/maxresdefault.jpg
/GettyImages-1091303816-601d1b45790b495d86be2a6522ea7b42.jpg)
Wash The Teeth Order Online Save 51 Jlcatj gob mx
https://www.verywellhealth.com/thmb/BgZn9aJVes-t_-ZEHwTCPbf5Oog=/1961x1529/filters:fill(87E3EF,1)/GettyImages-1091303816-601d1b45790b495d86be2a6522ea7b42.jpg
Molarity is the most commonly used method of concentration It is expressed as the number of moles of solute dissolved per litre of solution Therefore the unit of the molarity is mol L Molarity is also known as molar concentration and is represented by M The molarity or molar concentration of a solute is defined as the number of moles of solute per liter of solution not per liter of solvent What is a mole this video on the mole and Avogadro s number Molarity mol solute L of solution Why is the volume of the solution different from the volume of the solvent
You can convert from molarity M to normality N using the following equation N M n where n is the number of equivalents Note that for some chemical species N and M are the same n is 1 The conversion only matters when ionization changes the number of equivalents How Normality Can Change Molar refers to the unit of concentration molarity which is equal to the number of moles per liter of a solution In chemistry the term most often refers to molar concentration of a solute in a solution
More picture related to 1 molar is equal to

Prot sico Dental Dental Works Dental Life Dental Facts Dental Teeth
https://i.pinimg.com/originals/d3/cd/64/d3cd6425f9f2d3439a46fc7ca09839a7.jpg

Why Replace A Back Molar Marietta Tooth Replacement Muskingum
https://www.mvalleyoralsurgery.com/blog/wp-content/uploads/2020/07/AdobeStock_205778774__1582761439_20250-768x513.jpg

Molar Cavity Filling With Composite Dental Clinic
https://i.ytimg.com/vi/ercfRomycUA/maxresdefault.jpg
The most common way to express solution concentration is molarity M which is defined as the amount of solute in moles divided by the volume of solution in liters M moles of solute liters of solution Molarity is a unit of concentration measuring the number of moles of a solute per liter of solution The strategy for solving molarity problems is fairly simple This outlines a straightforward method to calculate the molarity of a solution An Example of How to Calculate Molarity
[desc-10] [desc-11]

Extraction Timing Of Heavily Destructed Upper First Permanent Molars
https://www.scirp.org/html/9-1460386x/cf785a7d-5d36-4b49-96a1-628ce72b6715.jpg
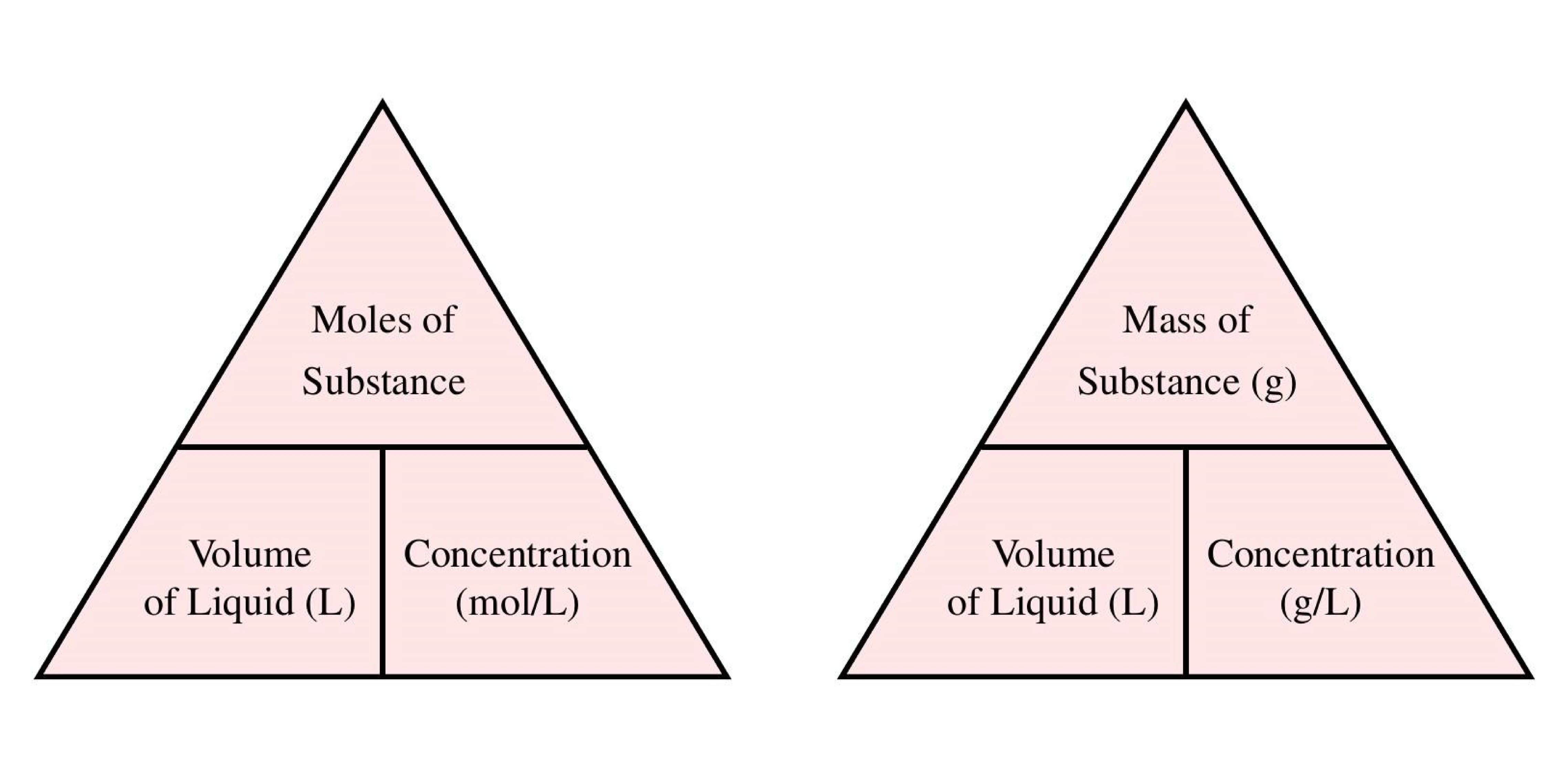
Calculate Molarity With Grams
https://www.ncl.ac.uk/webtemplate/ask-assets/external/maths-resources/images/Concentration_formula_triangles_2.png
1 molar is equal to - Molarity is the most commonly used method of concentration It is expressed as the number of moles of solute dissolved per litre of solution Therefore the unit of the molarity is mol L Molarity is also known as molar concentration and is represented by M