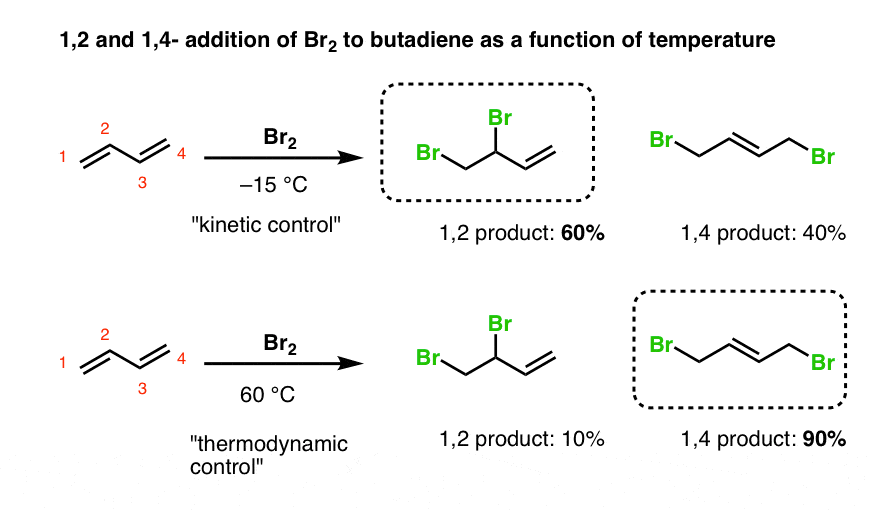
1 2 vs 1 4 product The 1 2 addition has a smaller activation energy than 1 4 addition it occurs faster than 1 4 addition because the bromide nucleophile is closer to carbon 2 then to carbon 4 However the 1 4 product is more stable than the 1 2 product
The more stable product is called a 1 4 adduct and the less stable product is the 1 2 adduct They are formed by a 1 4 and 1 2 addtion respectively The 1 4 adduct is the thermodynamic product of the reaction since it is the more stable product 16 8 Electrophilic Addition 1 2 Versus 1 4 Addition Page ID Layne Morsch University of Illinois Springfield Objectives After completing this section you should be able to write an equation for the addition of one or two mole equivalents of a halogen or a hydrogen halide to a nonconjugated diene
1 2 vs 1 4 product
1 2 vs 1 4 product
http://cdn.masterorganicchemistry.com/wp-content/uploads/2019/12/5-12-and-14-addition-of-br2-to-butadiene-is-a-function-of-temperature-at-low-temperature-minus-15-the-12-dominates-at-60-degrees-thermodnamic-dominates-to-give-14-addition.gif
2 Vs 1 YouTube
https://i.ytimg.com/vi/Rvf21jKyqDk/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AHOBYACgAqKAgwIABABGCIgZihyMA8=&rs=AOn4CLCrib9D8ES8klaXV2YqPq9Nm0PLXg

2 Vs 1 YouTube
https://i.ytimg.com/vi/eiBDEWleSDw/maxres2.jpg?sqp=-oaymwEoCIAKENAF8quKqQMcGADwAQH4Ac4FgAKACooCDAgAEAEYFyBSKH8wDw==&rs=AOn4CLDy8RknKXbnvAcVqt9Feva48NSw0A
The two different products are the 1 2 addition product where the H and Br are on adjacent carbons and the 1 4 addition product where the carbons bearing H and Br are separated by a double bond Temperature plays a key role in determining the product distribution 1 2 vs 1 4 addition Whether 1 2 or 1 4 addition occurs depends on multiple variables but mostly it is determined by the nature of the nucleophile During the addition of a nucleophile there is a competition between 1 2 and 1 4 addition products
Whether more 1 2 addition or 1 4 addition product is created depends largely on the temperature at which the reaction is run For example the addition of hydrogen bromide to 1 3 butadiene at temperatures below zero leads mainly to the 1 2 addition product while addition reactions run at temperatures above 50 C with these chemicals Carefully defines what is meant by 1 2 and 1 4 addition to dienes using bromination as a template reaction Looks at an example with one possible set of products from a single
More picture related to 1 2 vs 1 4 product

2 Vs 1 And I Still Ended Up Winning YouTube
https://i.ytimg.com/vi/1LIIpA-clrs/maxresdefault.jpg

2 Vs 1 NP D YouTube
https://i.ytimg.com/vi/l1vBs3JTOog/maxresdefault.jpg

2 Vs 1 YouTube
https://i.ytimg.com/vi/BbevuFgSMvM/maxres2.jpg?sqp=-oaymwEoCIAKENAF8quKqQMcGADwAQH4Ac4FgAKACooCDAgAEAEYZSBbKEAwDw==&rs=AOn4CLDhzOti9nQr6mB4CIG6-x1z9DBifg
1 2 product is achieved when the double bond gets protonated by HX carbocation is formed Then X attaches to the carbocation In another scenario when the carbocation is formed resonance can occur shifting the carbocation and double bond The end result is 1 4 product Mayya s Trick Since 1 2 additions to the carbonyl group are fast we would expect to find a predominance of 1 2 products from these reactions If the nucleophile is a weak base such as alcohols or amines then the 1 2 addition is usually reversible This means the competition between 1 2 and 1 4 addition is under thermodynamic control
1 2 vs 1 4 Addition Electrophilic addition in conjugated dienes gives a mixture of products 1 2 addition product addition to one of the double bonds occurs when the electrophile adds to one of the two adjacent carbons of the diene This organic chemistry video tutorial provides a basic introduction into the 1 2 addition reaction and the 1 4 addition of HBr to 1 3 butadiene The kinetic product is the major

1 Vs 1 2 Vs 2 Subs YouTube
https://i.ytimg.com/vi/z4mpYG2neeg/maxresdefault.jpg

1 4 Vs 3 8 Vs 1 2 Impact Driver Which One Is Better
https://99powertools.com/wp-content/uploads/2021/11/1-4-vs-3-8-vs-1-2-impact-driver-1-845x550.png
1 2 vs 1 4 product - One of the factors that decide the predominant product is the nature of the nucleophile Generally stronger nucleophiles such as lithium aluminum hydride Grignard reagents and organolithium reagents favor 1 2 addition However Grignard reagents can also yield a mixture of products