what percentage is 1 m hcl 1 g of HCl will be equal to 1 36 46 moles 444 01776 grams of Hydrogen chloride will be equal to 444 01776 x 1 36 46 12 178 moles Therefore we can say that 1 liter of Hydrochloric acid contains 12 178 moles of HCl or in other words
Step 1 Mass of the solution to make the 35 pure Let us assume that we have to prepare 1 L of 1 mol L 1 HCl 1 M represents the molarity number of moles of solute per litre of solution of X 1 1 12 2 x 0 082ml ml of water x 82mL HCl per liter To prepare a 1 M molar solution of hydrochloric acid HCl you would need to add 36 5 grams of hydrochloric
what percentage is 1 m hcl

what percentage is 1 m hcl
https://pharmabeej.com/wp-content/uploads/2023/04/0.01N-HCl-Preparation-1-1024x576.png
Circle Chart Set With Percentage And Pie Chart Set With 2 3 4 5 6 7 8 9
https://as2.ftcdn.net/v2/jpg/01/87/19/37/1000_F_187193756_HK8V5VKTYvIN2eUclxa4Q37ntQEJnOIv.jpg
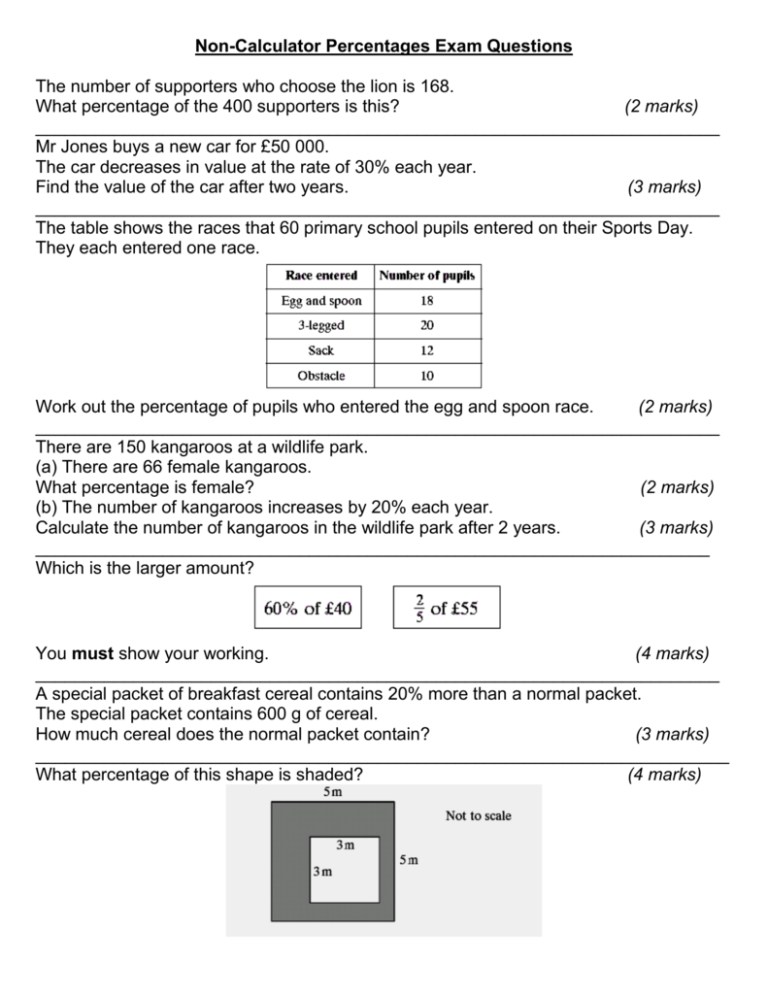
Non Calculator Percentages Exam Questions
https://s3.studylib.net/store/data/007684669_2-39ca470dd5564081121aade3c8a9e607-768x994.png
Thus a 1 M solution of H 2 SO 4 will be 2 N The normality of a solution is the molarity multiplied by the number of equivalents per mole Why does the calculator use 56 6 weight The molecular weight of KNO 3 is 101 1 g mol How much potassium nitrate should you weigh out First rearrange the molarity equation to solve for the mass of solute m m M MW V
There is no easy way to transform the mass percentage of a solution into molarity This is because percentage relies on the relative mass ratios while molarity is a measure per volume Common acid solutions can be prepared using the handy table below The third column lists the amount of solute acid that is used to make 1 L of acid solution Adjust the recipes accordingly to make larger or smaller
More picture related to what percentage is 1 m hcl

How Many ML Of 0 1 M HCl Are Required To React Completely With 1 G
https://dwes9vv9u0550.cloudfront.net/images/5468271/2c1d2320-5e1f-4967-b886-1e97b686d568.jpg
Body Fat Percentage By Picture For Men MennoHenselmans
https://mennohenselmans.com/wp-content/uploads/2023/02/bodyfat-percentage-compilation-men-front-1.png
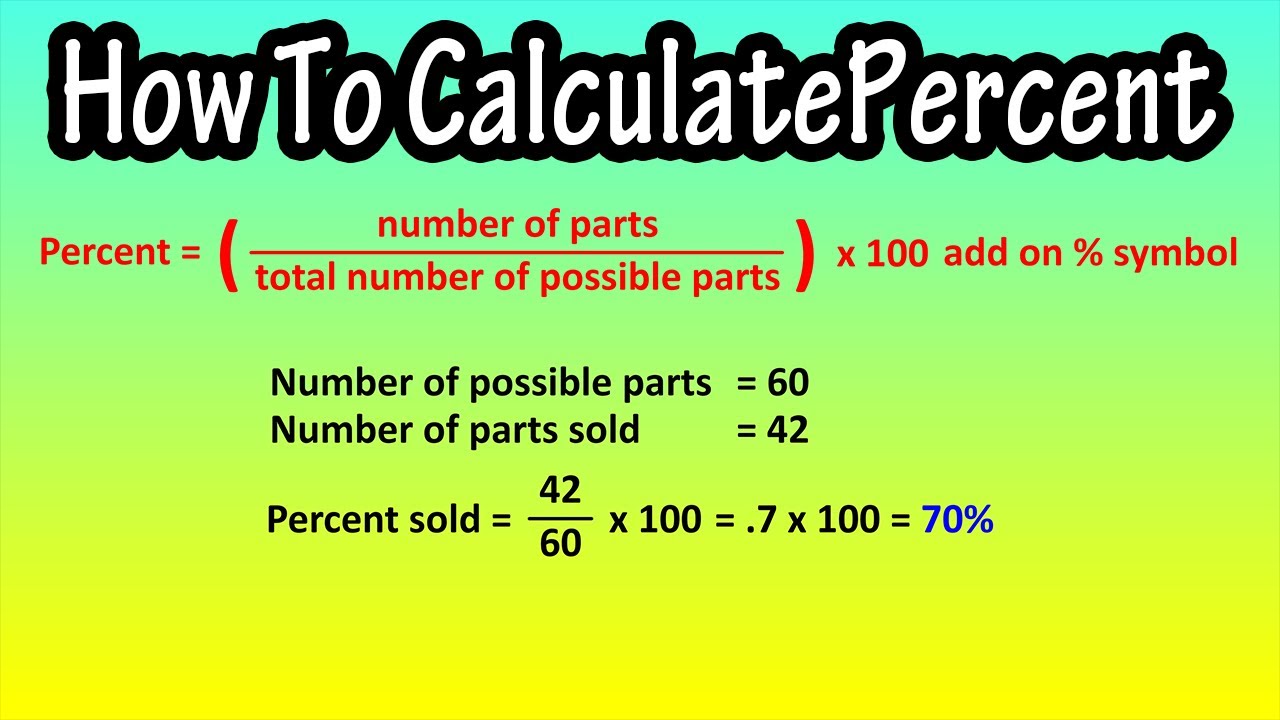
How To Calculate Percent Or Percentage Explained Formula For Percent
https://i.ytimg.com/vi/T3BwIvxSt_Q/maxresdefault.jpg
Example 35 ml of 1 25 M HCl acid is needed to titrate a 25 ml solution of NaOH In that case you can use the 1 1 formula because one mole of HCl reacts with one mole of NaOH Then multiply the molarity of the acid by Step 1 First convert the mass of solute to moles using the molar mass of HCl 36 5 g mol 22 4 gHCl 1 molHCl 36 5 gHCl 0 614mol HCl 22 4 g H C l 1 m o l H C l 36 5 g H C l 0 614 m o l H C l Step 2 Now we can use the
Dilution calculator molarity percent Each calculator cell shown below corresponds to a term in the formula presented above Enter appropriate values in all cells Hydrochloric acid abbreviation HCl aq is a common acid both in the body and in the lab It is for example a major component of gastric acid pH 1 2 0 5 w v HCl In

Using A Calculator How To Do Percentages For School Work
https://www.wikihow.com/images/1/1f/Do-Percentages-on-a-Calculator-Step-14-Version-2.jpg
How To Prepare 0 1 M HCL Solution Solution Parmacy
https://solutionpharmacy.in/wp-content/uploads/2023/04/How-to-Prepare-0.1-M-HCL-Solution-768x384.jpeg
what percentage is 1 m hcl - Find the normality of 0 321 g sodium carbonate in a 250 mL solution To solve this problem you need to know the formula for sodium carbonate Once you realize there are two