what is the unit of molecular weight When the molecular weight is given with the unit Da it is frequently as a weighted average similar to the molar mass but with different units In molecular biology the mass of macromolecules is referred to as their molecular weight and is expressed in kDa although the numerical value is often approximate and representative of an average
Molecular weight is commonly abbreviated by M W or MW Molecular weight is either unitless or expressed in terms of atomic mass units amu or Daltons Da Both atomic weight and molecular weight are defined relative to the mass of the isotope carbon 12 which is assigned a value of 12 amu In chemistry the molar mass M sometimes called molecular weight or formula weight but see related quantities for usage of a chemical compound is defined as the ratio between the mass and the amount of substance measured in
what is the unit of molecular weight

what is the unit of molecular weight
https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjEnaJ8PZsrnehXnpvdgLPcgaIWksbl3vW645ITTM7jruY4DZB2Z-55EYZ_0nJF0KJ7ZuUZ4LSkaV9iqyomeHy7zksf897vwzWX2jDOCl6rf4VwgRb6L-bF4Npo9fJ27OQ4jlv6C02Hyfq7Wwqt6f8hzadyCw-_udl3IX-hm9-Ff1otpLnZB0NDq5By/s16000/The molecular weight of hydrogen peroxide is 34. What is the unit of molecular weight [MP PMT 1986].jpg
Molecular Weight Explained In Plain English Jmhmanufacturing
https://jmhmanufacturing.com/wp-content/uploads/sites/130/2020/09/Article-22-molecular-weight-calculator.jpg

Molecular Weight Stoichiometry MCAT Content
https://i0.wp.com/cms.jackwestin.com/wp-content/uploads/2020/02/Screen-Shot-2020-02-08-at-7.52.31-PM.png?resize=1025%2C264&ssl=1
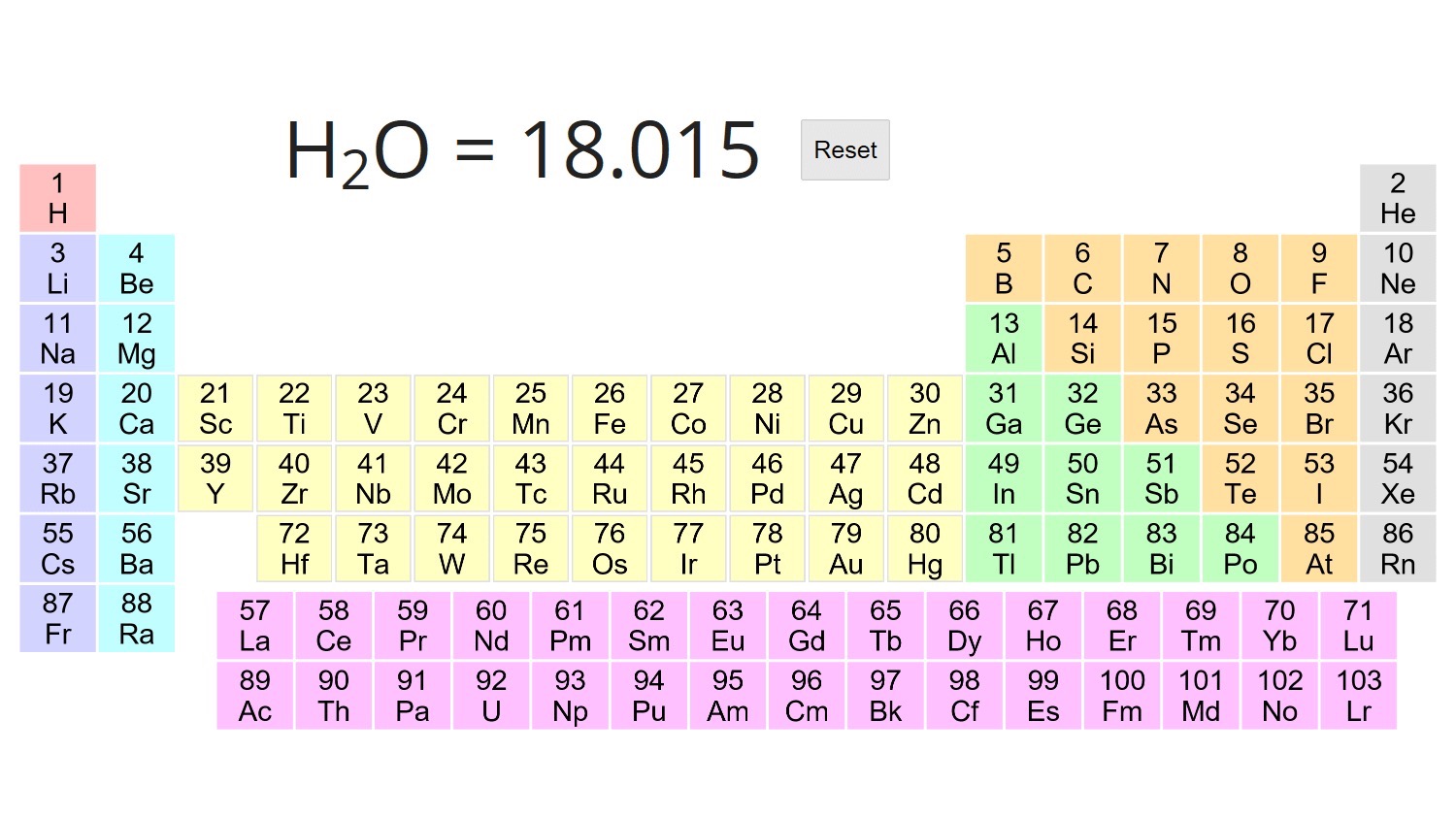
Molecular weight mass of a molecule of a substance based on 12 as the atomic weight of carbon 12 It is calculated in practice by summing the atomic weights of the atoms making up the substance s molecular formula The concept that allows us to bridge these two scales is molar mass Molar mass is defined as the mass in grams of one mole of a substance The units of molar mass are grams per mole abbreviated as g mol
First molecular mass is either unitless or else reported in daltons Da or atomic mass units amu or u On the other hand the unit for molar mass is grams per mole g mol or kilograms per mole kg mol Second molecular mass describes the mass of a single molecule or type of molecule The molar mass of a substance is defined as the mass of 1 mol of that substance expressed in grams per mole and is equal to the mass of 6 022 10 23 atoms molecules or formula units of that substance
More picture related to what is the unit of molecular weight
Newton unit Wikiwand
https://wikiwandv2-19431.kxcdn.com/_next/image?url=https:%2F%2Fupload.wikimedia.org%2Fwikipedia%2Fcommons%2Fthumb%2Fa%2Fad%2FOne_Newton%252C_illustrated_%2528transparent_background%2529.svg%2F1500px-One_Newton%252C_illustrated_%2528transparent_background%2529.svg.png&w=1080&q=50

Unit Campestre al gov br
https://d1whtlypfis84e.cloudfront.net/guides/wp-content/uploads/2020/12/15111415/Sample-10-1024x724.png
Ratios Rates Other Quiz Quizizz
https://quizizz.com/media/resource/gs/quizizz-media/quizzes/3bc06969-81e7-4b29-a0ca-9b6f24d07c40
Molecular mass is a dimensionless quantity but it is given the unit Dalton or atomic mass unit as a means of indicating the mass is relative to 1 12th the mass of a single atom of carbon 12 Also Known As Molecular mass is also called molecular weight The molar mass of an element is equal to its atomic mass in atomic mass units text amu converted to grams per mole For example the molar mass of carbon is 12 01 text g mol which is the atomic mass of carbon 12 01 text amu converted to grams per mole
[desc-10] [desc-11]
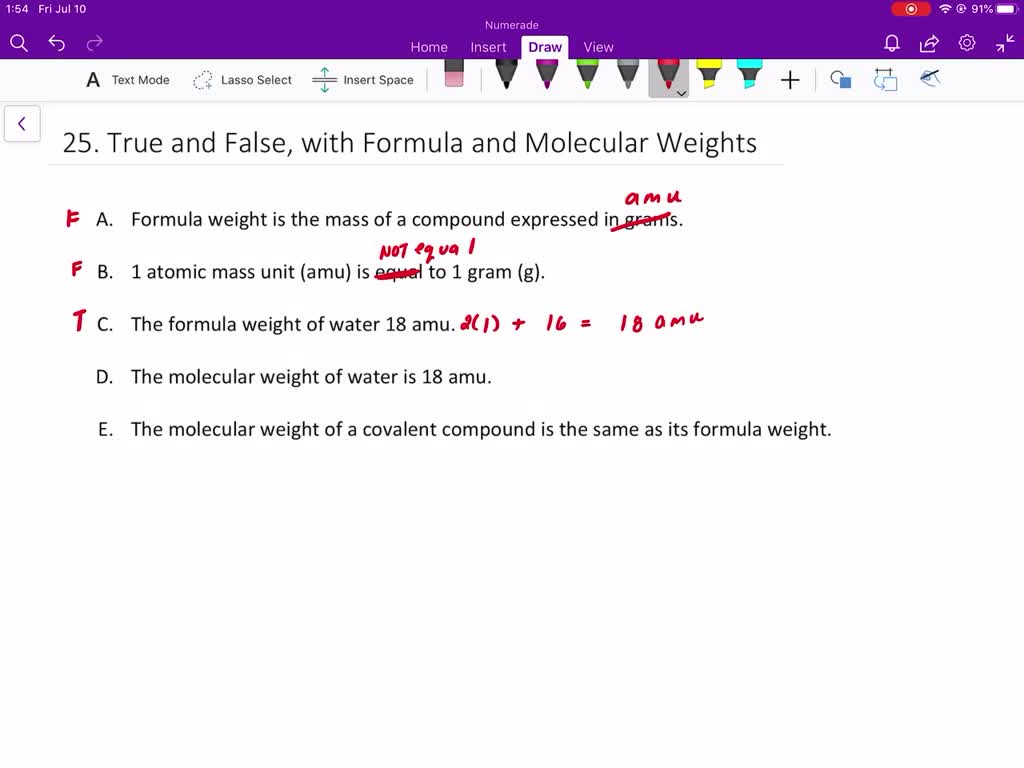
SOLVED Answer True Or False a Formula Weight Is The Mass Of A
https://cdn.numerade.com/previews/40e9f390-7756-4e66-9f24-f6ffe95bc36c_large.jpg

Which Of These Is The Unit Of Weight
https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/10945243.webp
what is the unit of molecular weight - [desc-12]