what is the half life of iodine 131 Half life 8 06 days Mode of decay Beta particles and gamma radiation Chemical properties I 131 can change directly from a solid into a gas skipping the liquid phase in a process called sublimation I 131 dissolves easily in water or alcohol
Iodine I 131 is a radioactive isotope of iodine with an atomic mass of 131 a half life of eight days and potential antineoplastic activity Selectively accumulating in the thyroid gland iodine I 131 emits beta and gamma particles thereby killing thyroid cells and decreasing thyroid hormone production Figure PageIndex 1 The half life of iodine 131 is eight days Half of a given sample of iodine 131 decays after each eight day time period elapses Half lives have a very wide range from billions of years to fractions of a second
what is the half life of iodine 131

what is the half life of iodine 131
https://hi-static.z-dn.net/files/d16/ef9196e37af9368d9b4f64d48439adf9.jpg

Solved Half life Iodine 131 A Radioactive Substance That Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/5f0/5f0e60e8-e50e-441f-84fa-a411021cef76/phpd1yThk.png
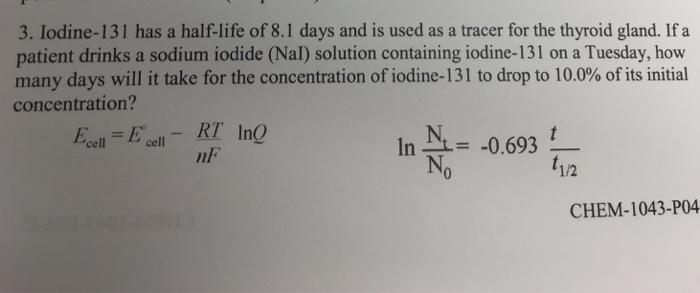
Solved Iodine 131 Is Radioactive And Has A Half Life Of 8 04 Chegg
https://media.cheggcdn.com/study/b6a/b6acc86b-fa51-41b4-9723-5449aa9e60b4/image.png
In essence the half life tells you at what time intervals you can expect an initial sample of a radioactive isotope to be halved In your case iodine 131 is said to have a half life of 8 days This means that with every 8 days that This iodine isotope has a physical half life of 8 0 days and emits particles with most abundant energy of 0 6 MeV and an average energy of 0 2 MeV and a range in tissue of 2 3 and 0 6 mm respectively
Iodine 131 has a half life of 8 02 days Calculate The number of iodine 131 atoms is initially present The activity of the iodine 131 in curies The number of iodine 131 atoms will remain in 50 days The time it will take for the activity to reach 0 1 mCi Solution The number of atoms of iodine 131 can be determined using isotopic mass as The radioisotopes used for imaging and treatment in medical sciences are usually synthesized and have a short half life so that they may not persist in the body for an unnecessarily long time For example phosphorous 32 iodine 131 and technetium 99m have half lives of 14 3 days 8 1 days and 6 0 hours respectively
More picture related to what is the half life of iodine 131
Half Life CK 12 Foundation
https://dr282zn36sxxg.cloudfront.net/datastreams/f-d%3Aa3729407f35ca46977c07c96e0cef1bc53a68f370c7bdba7dbdec09a%2BIMAGE_THUMB_POSTCARD%2BIMAGE_THUMB_POSTCARD.1
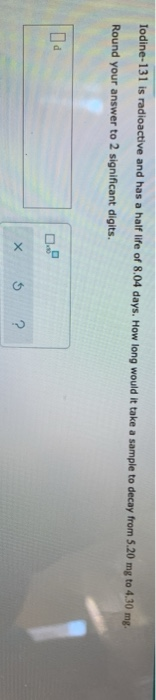
Solved Iodine 131 Has A Half life Of 8 1 Days And Is Used As Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/280/280833cd-5bb3-4218-9609-75051935b488/image
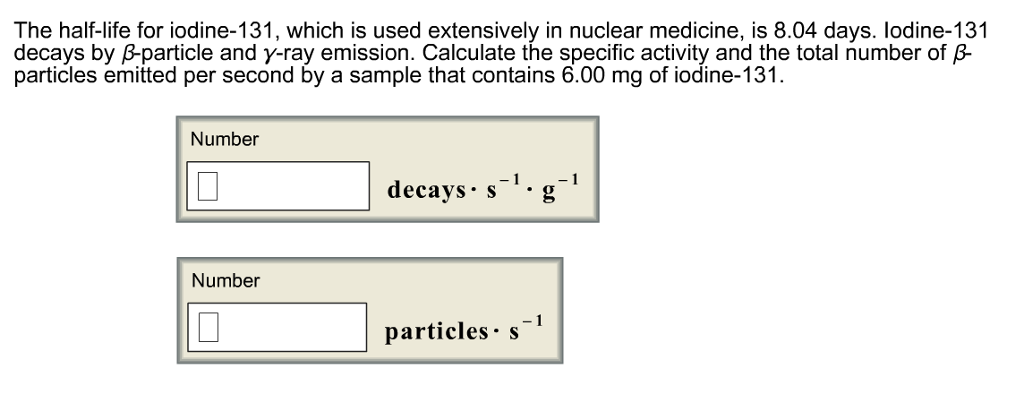
Solved The Half life For Iodine 131 Which Is Used Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/660/6602ec14-9373-4be1-908e-deec90cc6435/phpjM4obU.png
Iodine 131 s short half life of 8 days means that it will decay away completely in a matter of months Most I 129 in the environment came from nuclear weapons testing Atmospheric testing in the 1950s and 60s released radioactive iodine to the atmosphere Iodine 131 is a radioisotope with a very short period half life of 8 02 days making it highly radioactive Frequently used in small doses in thyroid cancers therapies it is also one of the most feared fission products when accidentally released into the
[desc-10] [desc-11]
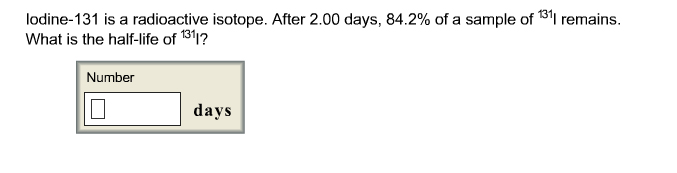
Solved Iodine 131 Is A Radioactive Isotope After 2 00 Days Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/b67/b67e2a3a-ce8a-4047-98cd-b13291bd8927/phpwUfEfS.png
SOLVED The Half life Of Iodine 131 Is 8 Days I Low Much Of A One Gram
https://cdn.numerade.com/previews/2203834f-b405-461e-8b70-f25514ad66cf_large.jpg
what is the half life of iodine 131 - The radioisotopes used for imaging and treatment in medical sciences are usually synthesized and have a short half life so that they may not persist in the body for an unnecessarily long time For example phosphorous 32 iodine 131 and technetium 99m have half lives of 14 3 days 8 1 days and 6 0 hours respectively