what is relative molecular mass The relative molecular mass Mr is the ratio of weighted average mass of a molecule of a molecular compound to the unified atomic mass unit The Mr has no units Mr The Mr can be found by adding up the relative atomic masses of
The relative molecular mass Mr is the ratio of weighted average mass of a molecule of a molecular compound to the unified atomic mass unit Mr has no units Mr can be found by adding up the relative atomic masses of all atoms present in one molecule You work out the relative molecular mass of a substance by adding up the relative atomic masses of the atoms it consists of So for example to work out the relative molecular mass of water H 2 O you add the relative atomic
what is relative molecular mass

what is relative molecular mass
https://i.ytimg.com/vi/hsMpLYVe77M/maxresdefault.jpg
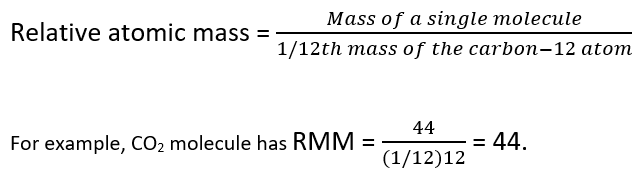
Relative Molecular Mass
https://alevelchemistry.co.uk/wp-content/uploads/2017/10/3-1.png
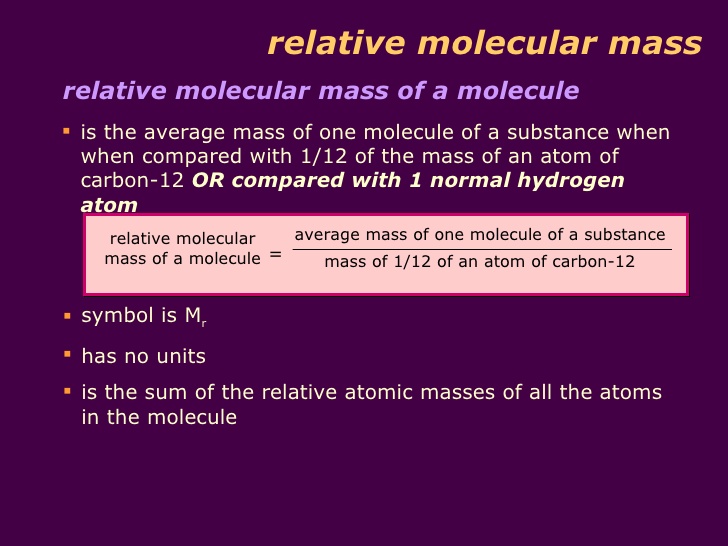
Relative Molecular Mass INSIDE CHEMISTRY
https://4.bp.blogspot.com/-_PasDvUkiFU/WMKPS5jSzAI/AAAAAAAAnUk/2UKAQhG5YMstilqwikzZ4LLJKtRovoJmACLcB/s1600/MR.jpg
The derived quantity relative molecular mass is the unitless ratio of the mass of a molecule to the atomic mass constant which is equal to one dalton The molecular mass and relative molecular mass are distinct from but related to the molar mass Relative molecular mass M r is the average mass of a molecule compared to one twelfth the mass of a single carbon 12 atom
The molecular mass gives the mass of a molecule relative to that of the 12 C atom which is taken to have a mass of 12 Molecular mass is a dimensionless quantity but it is given the unit Dalton or atomic mass unit as a means of indicating the mass is relative to 1 12th the mass of a single atom of carbon 12 Also Known As The molar mass of a substance is defined as the mass of 1 mol of that substance expressed in grams per mole and is equal to the mass of 6 022 10 23 atoms molecules or formula units of that substance
More picture related to what is relative molecular mass
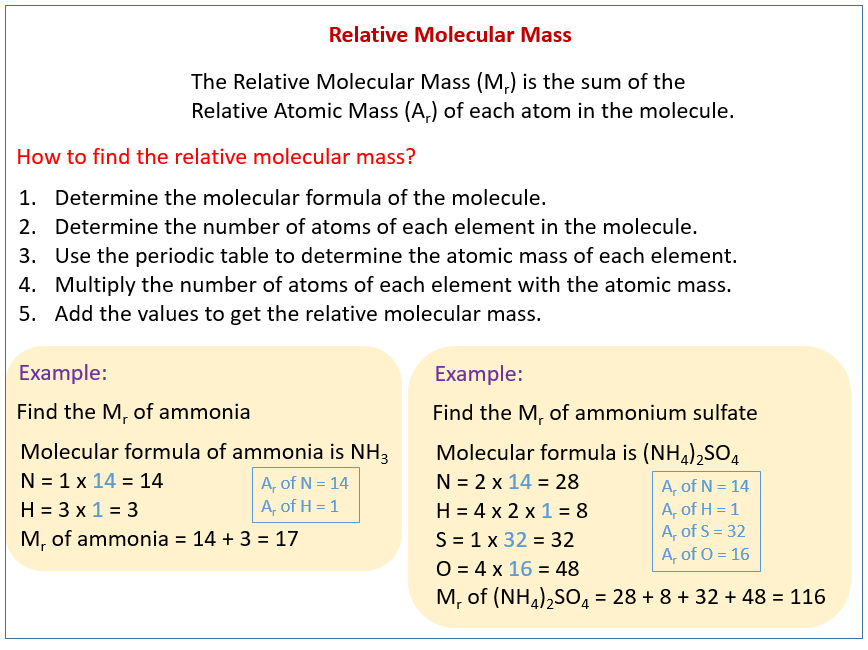
Relative Molecular Mass Relative Formula Mass solutions Examples
http://www.onlinemathlearning.com/image-files/xrelative-molecular-mass.png.pagespeed.ic.5gTup_RUNi.png

1 2 1 Define The Terms Relative Atomic Mass A R And Relative
https://i.ytimg.com/vi/hfT1DiMWRBA/maxresdefault.jpg

Relative Atomic Mass Molecular Mass O Level Chemistry Notes
https://i0.wp.com/chemnotcheem.com/wp-content/uploads/2020/04/comparing-the-relative-molecular-mass-and-molar-mass-of-water.png?fit=2736%2C1330&ssl=1
Summary The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units The formula mass of a covalent compound is also called the molecular mass In practice the relative molecular mass of a compound Mr is the sum of the relative atomic masses atomic weights of the atomic species as given in the chemical formula Chemical formula refers to either formula of an ionic compound formula of a molecule or molecular compound
[desc-10] [desc-11]

What Is Relative Atomic Mass Chemical Formula And Equation YouTube
https://i.ytimg.com/vi/IjtBbEkse1g/maxresdefault.jpg

Relative Molecular Mass
https://i.ytimg.com/vi/uIwqz7-a1BM/maxresdefault.jpg
what is relative molecular mass - The molecular mass gives the mass of a molecule relative to that of the 12 C atom which is taken to have a mass of 12 Molecular mass is a dimensionless quantity but it is given the unit Dalton or atomic mass unit as a means of indicating the mass is relative to 1 12th the mass of a single atom of carbon 12 Also Known As