what is collision theory Collision theory states that for a chemical reaction to occur the reacting particles must collide with one another The rate of the reaction depends on the frequency of collisions The theory also tells us that reacting particles often collide without reacting
Collision theory For a chemical reaction to occur the reactant molecules must collide with enough energy The minimum kinetic energy required for a reaction to occur is called the Collision theory is a principle of chemistry used to predict the rates of chemical reactions It states that when suitable particles of the reactant hit each other with the correct orientation only a certain amount of collisions result in a perceptible or notable change these successful changes are called successful collisions
what is collision theory

what is collision theory
https://d1e4pidl3fu268.cloudfront.net/66d30557-d3a8-466d-9255-3e4cd7e81613/Collisiontheory.png

Collision Theory Chemistry
https://s3-us-west-2.amazonaws.com/courses-images-archive-read-only/wp-content/uploads/sites/887/2015/08/23214431/CNX_Chem_12_05_COandO2.jpg

Collision Theory Cambridge O Level Chemistry Revision Notes 2023
https://cdn.savemyexams.com/cdn-cgi/image/f=auto,width=1920/uploads/2022/07/6-1-3-collision-theory-.png
According to the collision theory the molecules of reactants are assumed to be hard spheres and the reactions are assumed to occur only when these spheres molecules collide with each other What is collision theory Collision theory states that in order for a reaction to occur The particles must collide with each other The collision must have sufficient energy to cause a reaction i e enough energy to break bonds The minimum energy that colliding particles must have to react is known as the activation energy Collisions can be
Collision theory explains why different reactions occur at different rates and suggests ways to change the rate of a reaction Collision theory states that for a chemical reaction to occur the reacting particles must collide with one another What is Collision Theory The collision theory states that a chemical reaction can only occur between particles when they collide hit each other The collision between reactant particles is necessary but not sufficient for a reaction to take place
More picture related to what is collision theory
Collision Theory General Chemistry
https://s3-us-west-2.amazonaws.com/courses-images-archive-read-only/wp-content/uploads/sites/887/2015/04/23205758/CNX_Chem_12_05_COandO2.jpg
+Reacting+substances+must+collide+with+enough+energy+to+form+the+activated+complex..jpg)
Collision Theory Reaction Rates Ppt Download
https://slideplayer.com/slide/16861404/97/images/5/Collision+Theory+3)+Reacting+substances+must+collide+with+enough+energy+to+form+the+activated+complex..jpg

Kinetics Collision Theory A Level ChemistryStudent
http://www.chemistrystudent.com/images/ASPhysical/kinetics/collisiontheory2.png
Collision theory states that for a reaction to take place reactants must collide properly The rate of reaction is equal to the frequency of collisions Collision theory is limited to gases because frequencies of atomic collisions can only be calculated accurately with gases Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates The Arrhenius equation describes the relation between a reaction s rate constant and its activation energy temperature and dependence on collision orientation
[desc-10] [desc-11]
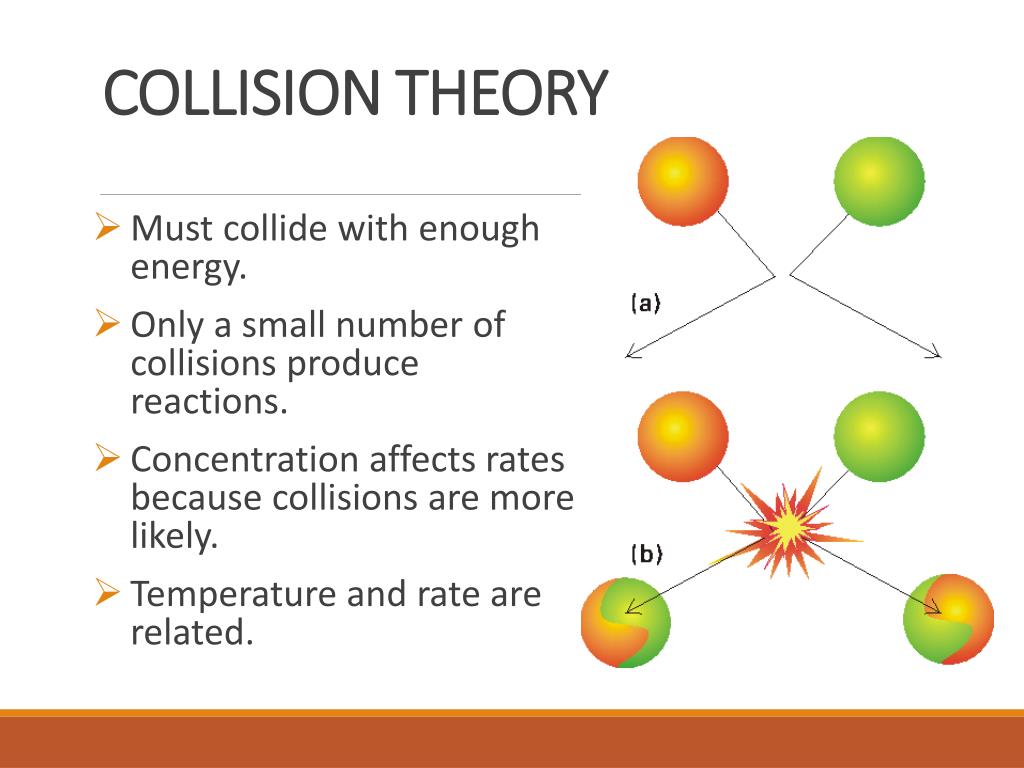
PPT KINETICS PowerPoint Presentation Free Download ID 1991748
https://image1.slideserve.com/1991748/collision-theory1-l.jpg
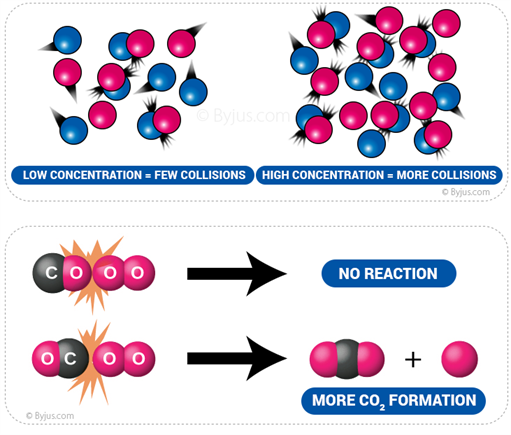
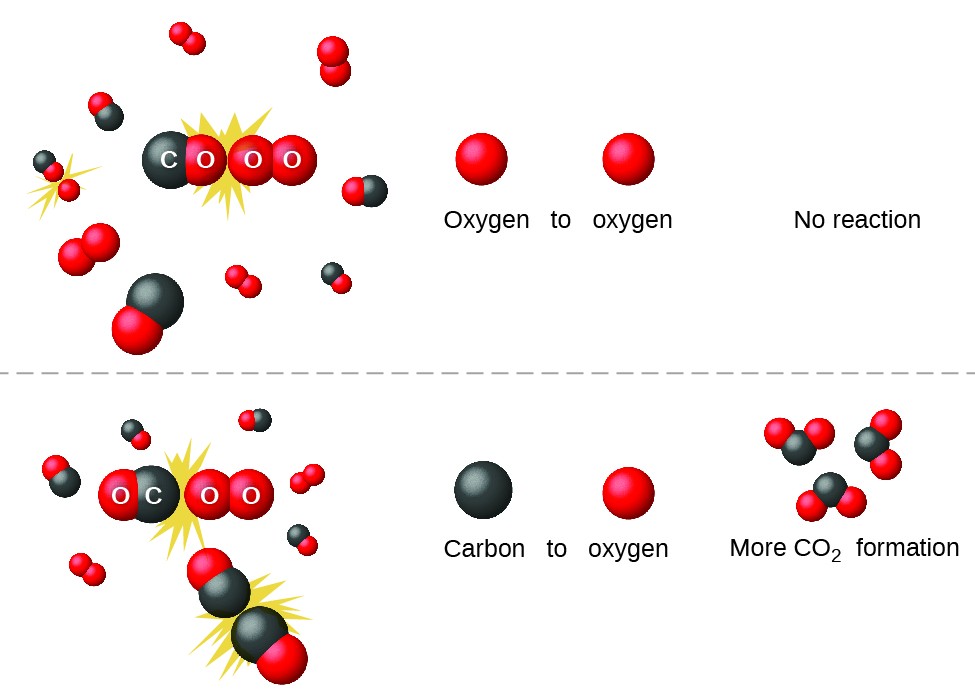
Collision Theory Molecular Collisions And Examples Chemistry Byju s
https://cdn1.byjus.com/wp-content/uploads/2018/11/chemistry/2017/08/06103758/Molecular-collisions.png
what is collision theory - What is collision theory Collision theory states that in order for a reaction to occur The particles must collide with each other The collision must have sufficient energy to cause a reaction i e enough energy to break bonds The minimum energy that colliding particles must have to react is known as the activation energy Collisions can be