what is average molecular mass The relative atomic mass average atomic mass as you put it is the weighted average mass of all the isotopes of an element in a given sample relative to the unified atomic mass unit which is defined as one twelfth of the mass of a carbon 12 atom in its ground state
The average molecular weight mass is the sum of the weight of all molecules divided by the total number of molecules that are present in that particular sample With the help of the molar composition and the molar weight The atomic mass unit u is a unit that describes the masses of individual atoms and molecules The atomic mass is the weighted average of the masses of all isotopes of an element The molecular mass is the sum of
what is average molecular mass
what is average molecular mass
https://cdn1.byjus.com/chemistry/wp-content/uploads/2016/01/slide_18.jpg

Atomic Mass Of Iron Julian Mackay
https://i.pinimg.com/736x/60/ba/e4/60bae4f6bc0279b7ef6525d328da66a4.jpg
:max_bytes(150000):strip_icc()/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Mass And Atomic Mass Number
https://www.thoughtco.com/thmb/vSpe3Hy58nZeJ4tZQ8_fComvX7I=/1500x0/filters:no_upscale():max_bytes(150000):strip_icc()/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png
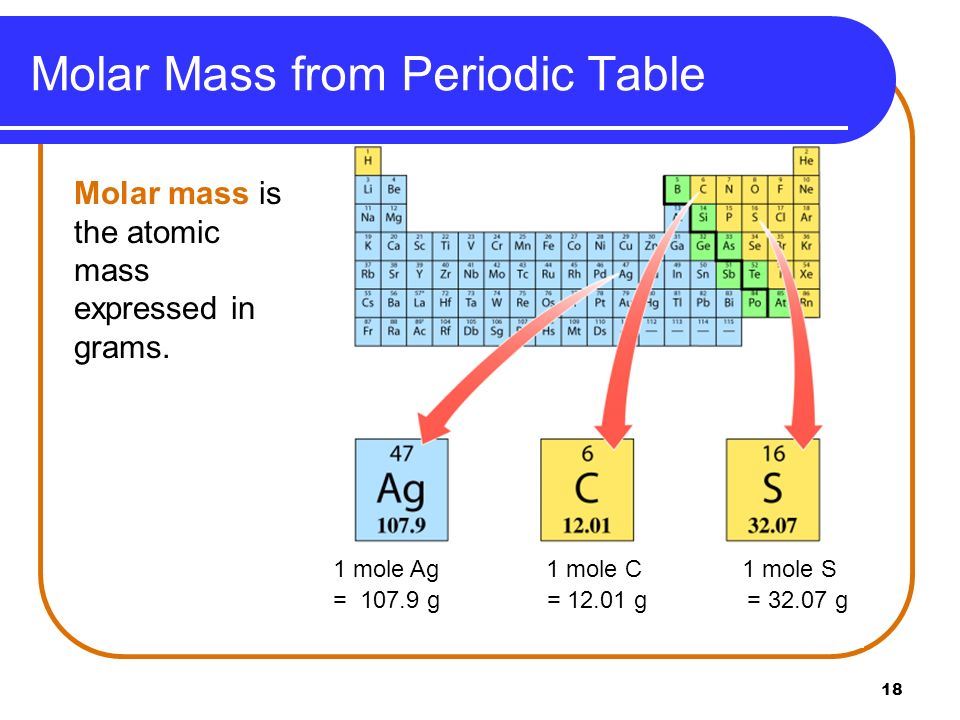
The molar mass of any substance is its atomic mass molecular mass or formula mass in grams per mole The periodic table lists the atomic mass of carbon as 12 011 amu the average molar mass of carbon the mass of 6 022 10 23 carbon atoms is therefore 12 011 g mol The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units The formula mass of a covalent compound is also called the molecular mass
The molar mass of any substance is numerically equivalent to its atomic or formula weight in amu Per the amu definition a single 12 C atom weighs 12 amu its atomic mass is 12 amu A mole of 12 C weighs 12 g its molar mass is 12 g mol Molecular mass is a number equal to the sum of the atomic masses of the atoms in a molecule The molecular mass gives the mass of a molecule relative to that of the 12 C atom which is taken to have a mass of 12
More picture related to what is average molecular mass

Mass Vs Weight Don t Get Weighed Down By Confusion ESLBUZZ
https://www.eslbuzz.com/wp-content/uploads/2023/07/how-to-measure-mass-vs-weight.png

In A Polymer Sample 30 Molecules Have Molecular Mass 20000 40 Have
https://i.ytimg.com/vi/sWaCDpsflGM/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLATD0fszvF5mlUOqlfFZy6qieTEtA

Introduction To Polymers Lecture 4 3 Weight Average Molecular
https://i.ytimg.com/vi/prO5gJSprFQ/maxresdefault.jpg
The molar mass of a substance is the mass in grams of 1 mole of the substance As shown in this video we can obtain a substance s molar mass by summing the molar masses of its component atoms We can then use the calculated molar mass to convert between mass and number of moles of the substance The molecular mass or molecular weight is the total mass of a compound It is equal to the sum of the individual atomic masses of each atom in the molecule It s easy to find the molecular mass of a compound with these steps Determine the molecular formula of the molecule
[desc-10] [desc-11]
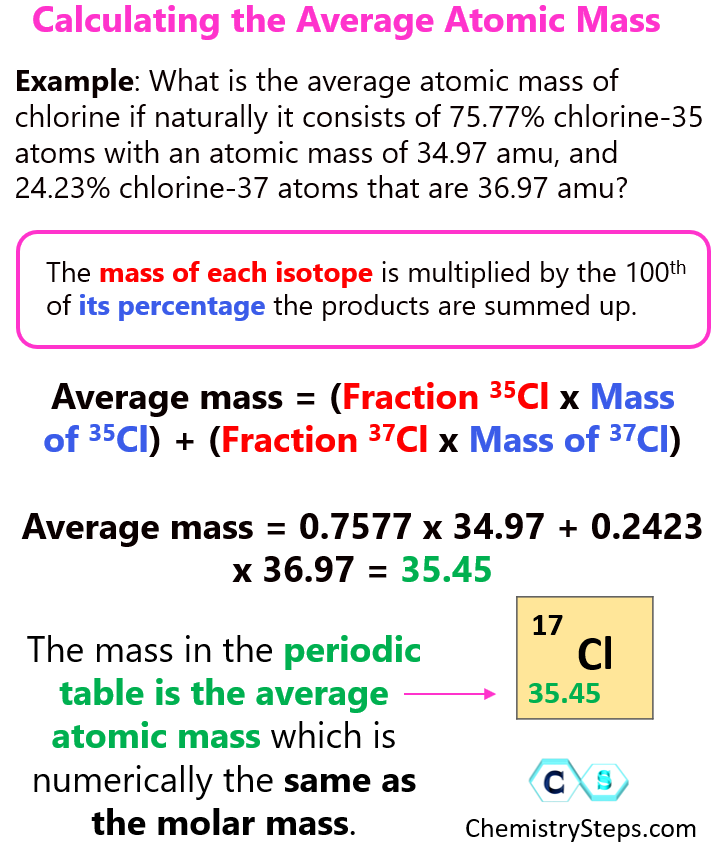
How To Calculate The Average Atomic Mass Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2023/01/How-To-Calculate-The-Average-Atomic-Mass.png

Number Average Molecular Mass M N Mass Average Molecular Mass M W
https://www.researchgate.net/profile/Marco_Contardi/publication/332277549/figure/download/tbl1/AS:745486495543297@1554749239082/Number-average-molecular-mass-M-n-mass-average-molecular-mass-M-w-and.png
what is average molecular mass - The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units The formula mass of a covalent compound is also called the molecular mass