what is an elementary reaction In chemistry an elementary reaction is a chemical reaction that proceeds in a single step with only one transition state reactants products An elementary reaction cannot be broken down into simpler reactions and generally has no intermediates
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form products in a single reaction step and with a single transition state An elementary reaction is a chemical reaction where reactants directly form products in a single step with a single transition state Here no intermediate products are formed The transition state is the phase of the reaction where the
what is an elementary reaction

what is an elementary reaction
https://sciencenotes.org/wp-content/uploads/2022/10/Elementary-Reaction-in-Chemistry-768x512.png

Reaction Mechanism Definition Elementary Steps Expii
https://d20khd7ddkh5ls.cloudfront.net/untitled-artwork_2_8.png
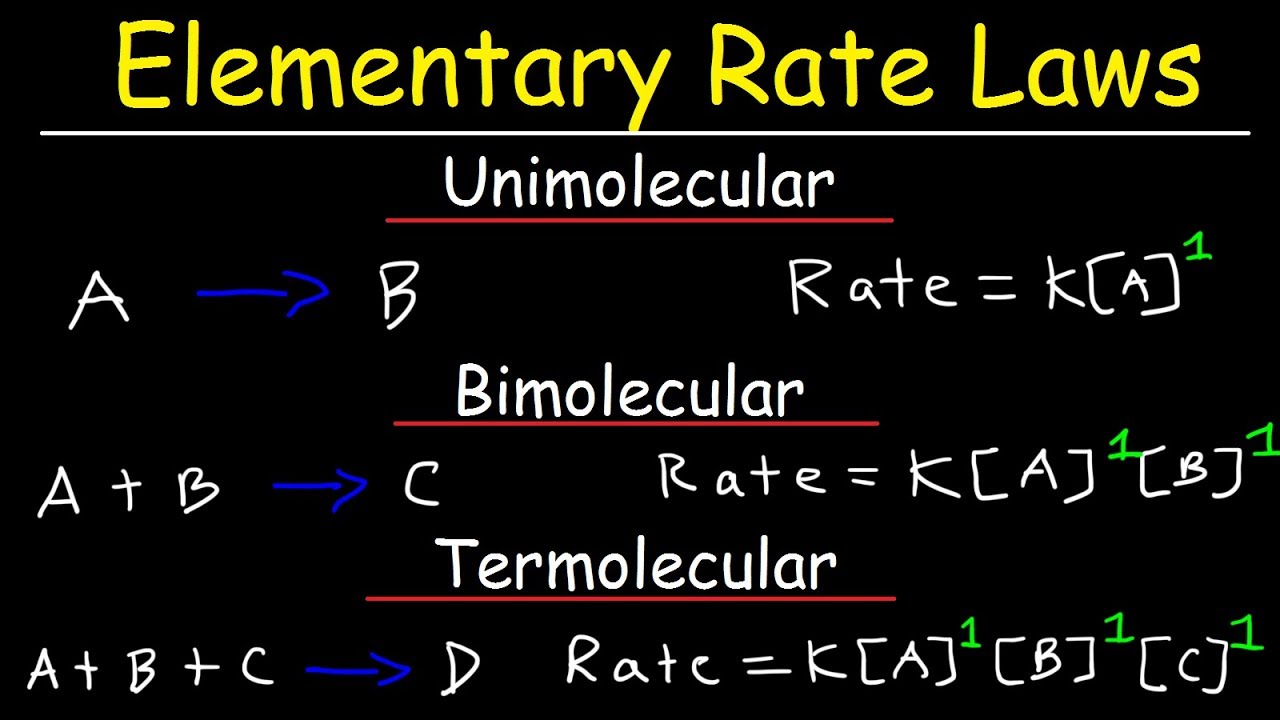
Elementary Rate Laws Unimolecular Bimolecular And Termolecular
https://i.ytimg.com/vi/S84Llf1vqiM/maxresdefault.jpg
An elementary reaction is a chemical reaction where reactants form products in a single step with a single transition state Elementary reactions may combine to form complex or nonelementary reactions An elementary reaction is a reaction that occurs in a single step The rate law for an elementary reaction can be derived from the coefficients of the reactants in the balanced equation For example the rate law for the elementary reaction 2A
An elementary reaction involves the collision breaking apart or rearrangement of atomic scale particles to form reaction products and or intermediates The equation of an elementary reaction precisely indicates the atoms or molecules participating in the reaction An elementary step or elementary reaction is one step in a series of simple reactions that show the progress of a reaction at the molecular level A reaction mechanism is the sequence of elementary steps that together comprise an entire chemical reaction
More picture related to what is an elementary reaction

Elementary And Non Elementary Reaction no 18 Copy
https://image.slidesharecdn.com/elementaryandnon-elementaryreactionno-18-copy-151029150600-lva1-app6891/95/elementary-and-non-elementary-reactionno18-copy-3-1024.jpg?cb=1446131301

Difference Between Elementary And Nonelementary Reaction YouTube
https://i.ytimg.com/vi/ROOStXUJ0mY/maxresdefault.jpg
What Is An Elementary Reaction
https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/9852537.webp
An elementary reaction shows which atomic scale particles collide break apart or rearrange their structures to form reaction products and or intermediates The equation for an elementary reaction specifies exactly which atoms or molecules are involved in that reaction A reaction that occurs in two or more elementary steps is called a multistep or complex reaction A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step The slowest step in a reaction mechanism is known as the rate determining step
[desc-10] [desc-11]
:max_bytes(150000):strip_icc()/GettyImages-85594625-56a135025f9b58b7d0bd0674.jpg)
Elementary Reaction Definition
https://www.thoughtco.com/thmb/FTEW9rIXG4-nsuV57vSJAK20lVk=/768x0/filters:no_upscale():max_bytes(150000):strip_icc()/GettyImages-85594625-56a135025f9b58b7d0bd0674.jpg
/radioactive-decay-dd02334f968143b1a2de5aa59fcc74b5.jpg)
Elementary Reaction Definition In Chemistry
https://www.thoughtco.com/thmb/KqUvZ2o76jIrkoj8FlyljRMGfUM=/1500x1000/filters:fill(auto,1)/radioactive-decay-dd02334f968143b1a2de5aa59fcc74b5.jpg
what is an elementary reaction - An elementary reaction involves the collision breaking apart or rearrangement of atomic scale particles to form reaction products and or intermediates The equation of an elementary reaction precisely indicates the atoms or molecules participating in the reaction