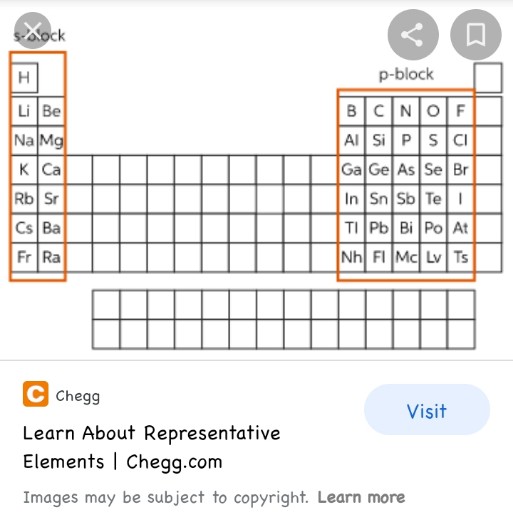
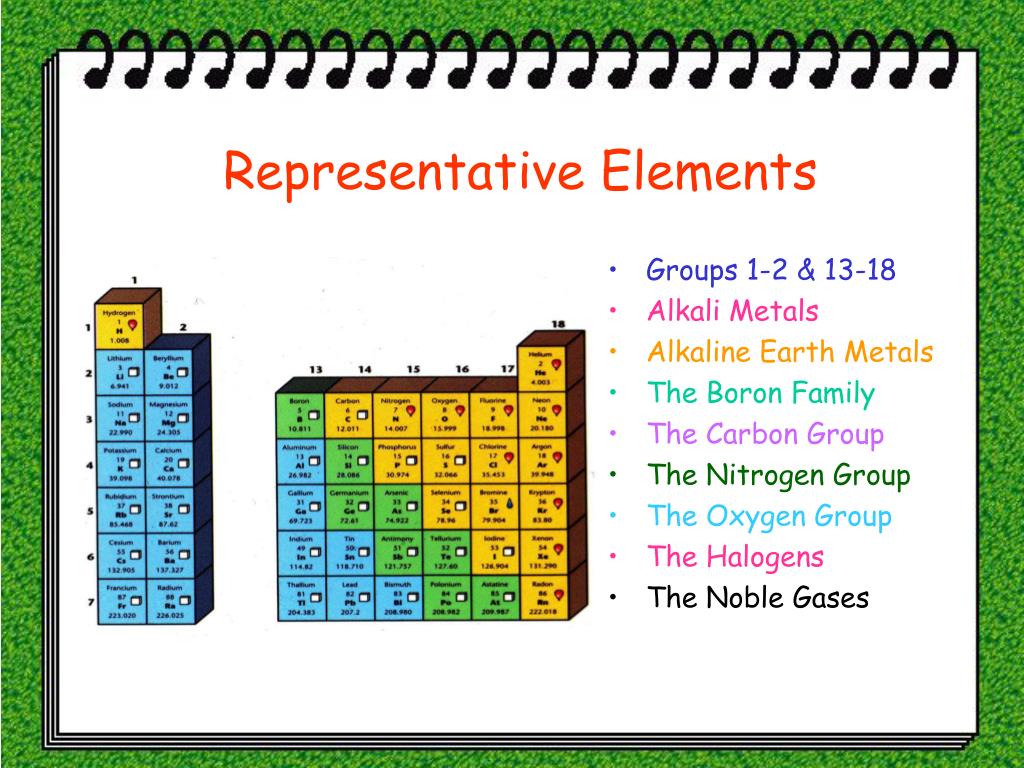
what is a representative elements What representative elements are can be understood by their position on the periodic table The location of Representative elements on the periodic table are in groups 1 2 and from 13 to 18
Representative Elements are also known as Group A S and P Block Elements or Main Group Elements Significance of the Layout The layout of the periodic table demonstrates recurring chemical properties Elements are listed in order of increasing atomic number the number of protons in the atomic nucleus and arranged This page titled 12 Chemistry of the Representative Elements is shared under a CC BY NC SA 4 0 license and was authored remixed and or curated by Ed Vitz John W Moore Justin Shorb Xavier Prat Resina Tim Wendorff Adam Hahn
what is a representative elements
what is a representative elements
https://4.bp.blogspot.com/-w-SC6AHJEv8/TZLmyfZe9II/AAAAAAAAA20/nL43ByyvX00/s1600/Dibujo.JPG

What Are The Transition Metals On The Periodic Table
https://sciencenotes.org/wp-content/uploads/2020/09/Element-Blocks.png
Representative Elements Why This Name Inorganic Chemistry Science
https://www.scienceforums.net/uploads/monthly_2021_07/Screenshot_2021_0725_235520.jpg.f67a06d7b3fa0d58d3c0416b27ef7a43.jpg
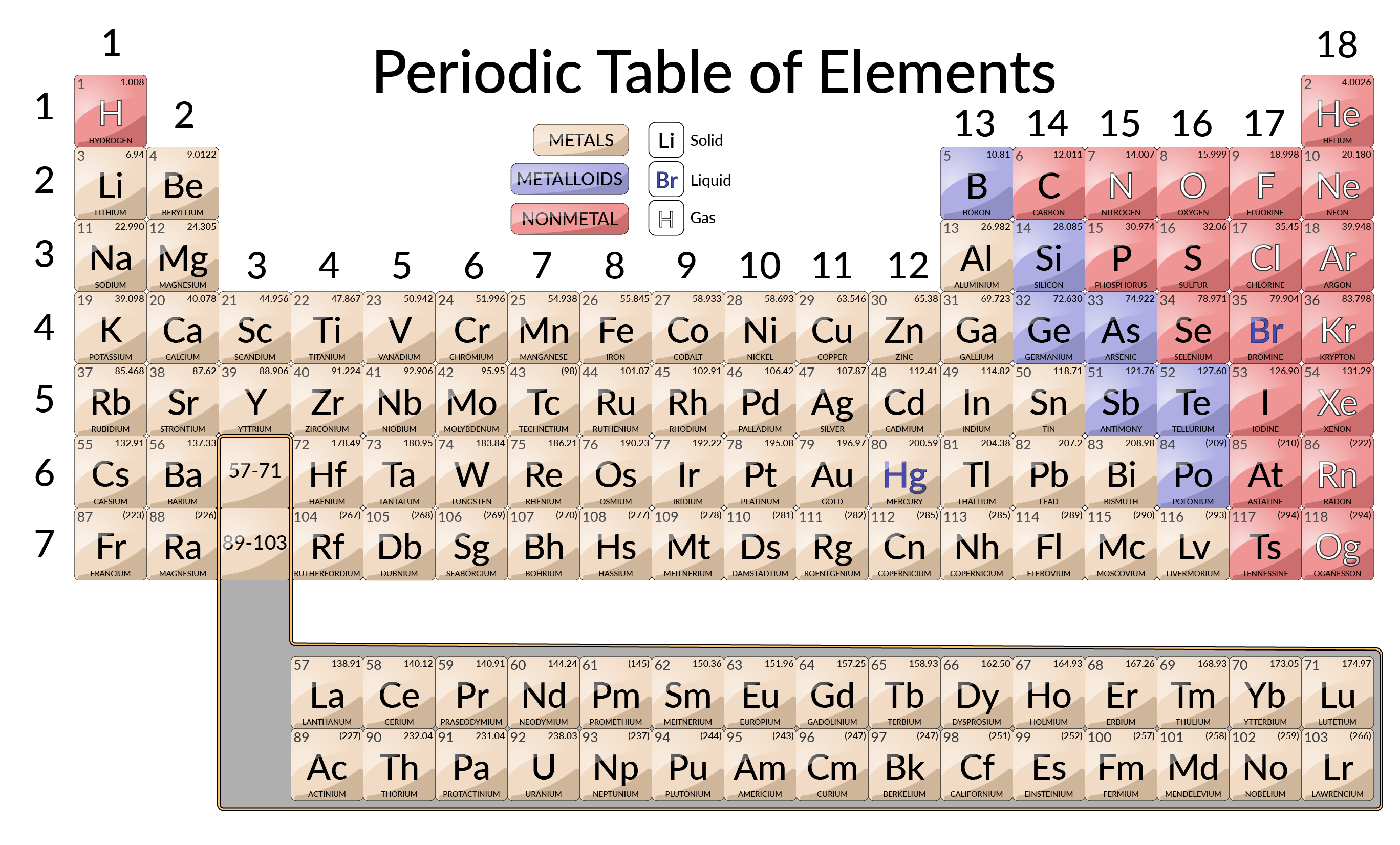
In chemistry and atomic physics the main group is the group of elements sometimes called the representative elements whose lightest members are represented by helium lithium beryllium boron carbon nitrogen oxygen and fluorine as arranged in the periodic table of the elements The representative elements occur in groups 1 2 and 12 18 These elements are representative metals metalloids and nonmetals The alkali metals group 1 are very reactive readily form ions with a charge of 1 to form ionic compounds that are usually soluble in water and react vigorously with water to form hydrogen gas and a
Representative elements themselves though the representative elements are the remaining elements not found in groups 3 to 12 so we re talking about groups 12 and then 13 to 18 They are sometimes referred to as our group a elements or our main group elements So if they re talking about group a elements main group elements all that The representative elements are the A elements found in groups IA VIIIA Representative elements have their valence electrons in orbitals of either s or p shells The roman numeral and the letter designation determine the electron configuration As an example an element in Group VA has five valence electrons with the configuration s 2 p
More picture related to what is a representative elements
Representative Elements Periodic Table
https://www.jove.com/files/core/CDNSource/lessons/806/806_Image_1_white.png
Representative Elements Periodic Table
https://unacademy.com/content/wp-content/uploads/sites/2/2022/04/u1-1.png
PPT The Periodic Table Of Elements PowerPoint Presentation Free
https://image3.slideserve.com/5845878/representative-elements-l.jpg
The representative elements or main group elements include all nonmetals and a few metals The 18th group elements or noble gases of the periodic table are not part of these representative elements because of their filled valence shells Also they do not tend to share or transfer electrons for bond formation The Inert Pair Effect The inert pair effect refers to the empirical observation that the heavier elements of groups 13 17 often have oxidation states that are lower by 2 than the maximum predicted for their group For example although an oxidation state of 3 is common for group 13 elements the heaviest element in group 13 thallium Tl is more
The main difference between representative and transition elements is that representative elements also known as main group elements are found in the s and p blocks of the periodic table whereas transition elements are located in the d and f blocks Representative and transition elements collectively form the essential building The elements can also be classified into the main group elements or representative elements in the columns labeled 1 2 and 13 18 the transition metals in the columns labeled 3 12 2 and inner transition metals in the two rows at the bottom of the table the top row elements are called lanthanides and the bottom row elements are

Representative Elements Are Those Which Belong To
https://www.gkseries.com/blog/wp-content/uploads/2022/04/Representative-elements-are-those-which-belong-to-1024x536.png

Which Of The Following Is Not A Representative Element YouTube
https://i.ytimg.com/vi/FkoA7c2NpZs/maxresdefault.jpg
what is a representative elements - Other articles where representative element is discussed chemical compound The periodic table and 2 are called the representative metals those in the centre of the periodic table are called the transition metals The lanthanoids and actinoids shown below the periodic table are special classes of transition metals