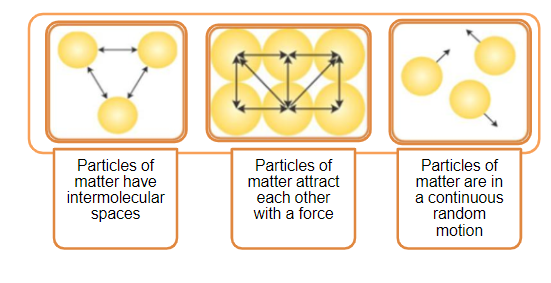
what is a postulate of the kinetic molecular theory The kinetic molecular theory KMT is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter This theory is
The Kinetic Molecular Theory Postulates The experimental observations about the behavior of gases discussed so far can be explained with a simple theoretical model known as the kinetic molecular theory This theory is based 5 Postulates of Kinetic Theory 1 Molecules move continuously and randomly in straight lines in all directions and various speeds Properties of a gas that depend on motion of molecules
what is a postulate of the kinetic molecular theory

what is a postulate of the kinetic molecular theory
https://i.ytimg.com/vi/dL4_ohvaU_k/maxresdefault.jpg
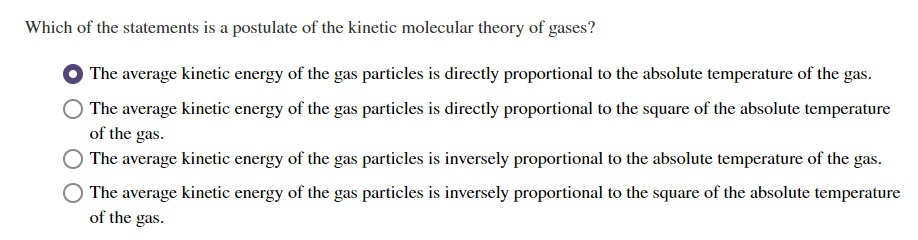
Solved Which Of The Statements Is A Postulate Of The Kinetic Chegg
https://media.cheggcdn.com/media/84b/84b22ee9-a21b-46e9-bc27-ed3f09896665/phpb5D8vj.png

The Kinetic Molecular Theory Of Gasses Matter Going To Tehran
https://i2.wp.com/goingtotehran.com/wp-content/uploads/2017/12/2.jpg?resize=650%2C320&ssl=1
The postulates of kinetic molecular theory also known as molecular kinetic theory start sensibly with Molecules and Moving Which can be expanded to Matter be molecules and The kinetic molecular theory of gases KMT or simply kinetic theory of gases is a theoretical model that explains the macroscopic properties of a gas using statistical mechanics These properties include the pressure
The kinetic molecular theory KMT is a simple microscopic model that effectively explains the gas laws described in the previous sections of this chapter This theory is based on the The kinetic molecular theory KMT is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter This theory is based on the following
More picture related to what is a postulate of the kinetic molecular theory
Definition Theorems And Postulates AAS Theorem Media4Math
https://www.media4math.com/sites/default/files/library_asset/images/GeometryTheorems--AASTheorem.png

The Postulates Of Kinetic Molecular Theory Real Chemistry YouTube
https://i.ytimg.com/vi/IZSNADrN9Rs/maxresdefault.jpg
What Are The Postulate Of Kinetic Theory Of Matter Ku7k0g
https://images.topperlearning.com/topper/tinymce/imagemanager/files/b0ee8ec00740fb847b2d4424c3a8371d5ec226bfaf7d81.23388143KinetictheoryofMatter.PNG
In order to apply the kinetic model of gases five assumptions are made Gases are made up of particles with no defined volume but with a defined mass In other words their volume is miniscule compared to the distance The kinetic molecular theory describes the properties of molecules in terms of motion kinetic energy and of temperature The theory is most often applied to gases but is helpful in
Kinetic molecular theory states that molecules have an energy of motion kinetic energy that depends on temperature Rudolf Clausius developed the kinetic theory of heat which relates The kinetic molecular theory KMT is a simple molecular model that effectively explains the physical properties of matter using the motion of the molecules This theory

Kinetic Molecular Theory Kinetic Molecular Theory The Kinetic
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/e222b6eaaa80271b49c13fcdf27332cd/thumb_1200_1749.png

Postulates Of Kinetic Molecular Theory Of Liquids Chemistry Lecture
https://i.ytimg.com/vi/Nk_ow1S0P30/maxresdefault.jpg
what is a postulate of the kinetic molecular theory - Using the postulates of the kinetic molecular theory explain why a gas uniformly fills a container of any shape Can the speed of a given molecule in a gas double at constant