What Is 21 Cfr 210 - Worksheets are currently essential tools used in a wide range of activities, including education, commerce, and personal monitoring. They supply structured styles that sustain learning, strategizing, and decision-making throughout different levels of intricacy, from basic mathematics troubles to detailed business examinations.
How Flosum Helps In Salesforce 21 CFR Part 11 Compliance Flosum DevOps

How Flosum Helps In Salesforce 21 CFR Part 11 Compliance Flosum DevOps
Worksheets are structured documents made use of to arrange information, details, or tasks systematically. They offer a graph of concepts, permitting individuals to input, control, and assess data successfully. Whether in the classroom, the boardroom, or at home, worksheets streamline procedures and boost productivity.
Kinds of Worksheets
Educational Worksheets
In educational settings, worksheets are indispensable resources for teachers and pupils alike. They can vary from mathematics issue sets to language comprehension exercises, supplying possibilities for method, support, and analysis.
Printable Service Equipments
In business globe, worksheets offer numerous functions, including budgeting, task preparation, and information analysis. From monetary declarations to SWOT analyses, worksheets aid services make notified choices and track development towards objectives.
Personal Worksheets
On an individual degree, worksheets can assist in goal setting, time management, and routine tracking. Whether preparing a spending plan, arranging an everyday routine, or checking physical fitness progress, personal worksheets supply structure and responsibility.
Making best use of Understanding: The Benefits of Worksheets
Worksheets offer various benefits. They boost involved learning, boost understanding, and nurture logical reasoning capacities. Additionally, worksheets support framework, boost effectiveness and allow teamwork in team circumstances.

21 CFR Food And Drugs CGMP Finished Pharmaceuticals

What Is 21 CFR Part 11 And Why Do You Need To Know More About It
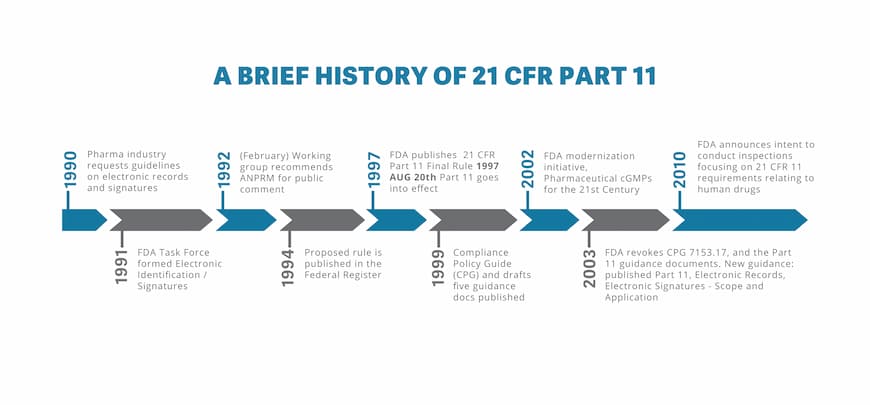
FDA 21 CFR Part 11 Explained Life Sciences ShareVault

What Is 21 CFR Part 11 MadgeTech
What Is FDA s 21 CFR Part 820

What Is 21 CFR Part 11 And Why Do You Need To Know More About It

FDA 21 CFR 210 And 211 Quality Assurance By Operon Strategist Medical
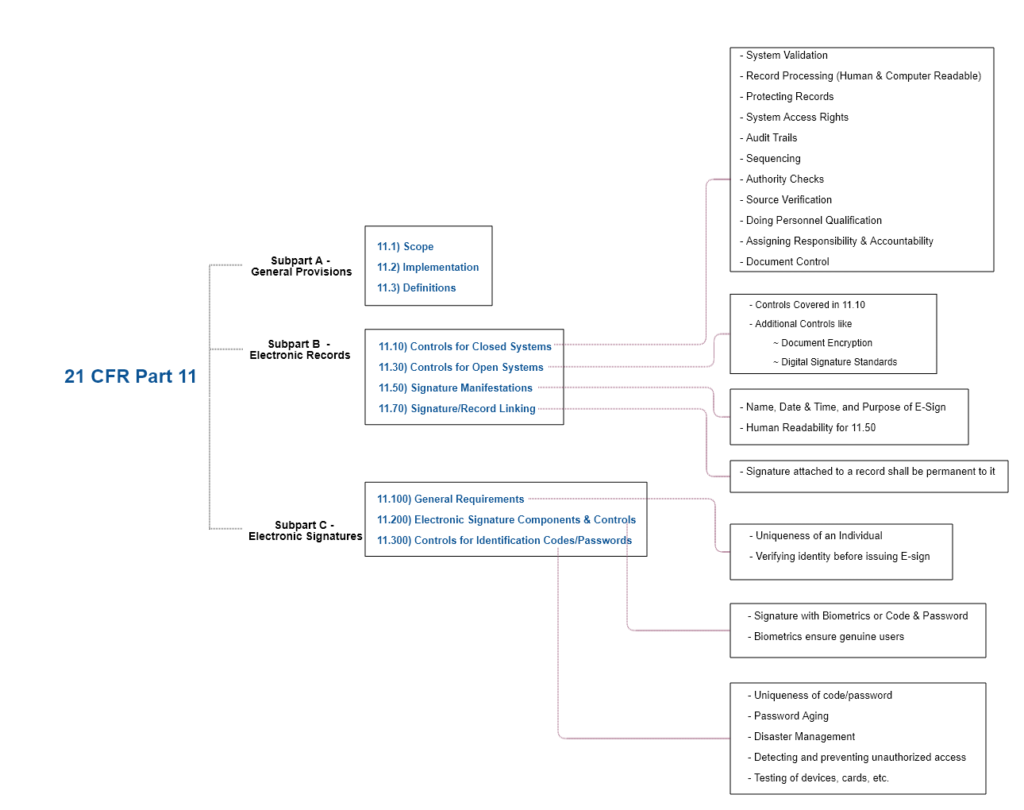
FDA s 21 CFR Part 11 The Definitive Guide Pharma GxP

Stay Compliant With FDA s 21 CFR Part 11 Regulation Cygnature

What Is 21 CFR Part 11 Do You Need To Comply With 21 CFR Part 11