what is 21 cfr 210 and 211 Current Good Manufacturing Practice in Manufacturing Processing packing or Holding of Drugs 21 CFR Part 211 Current Good Manufacturing
PART 211 CURRENT GOOD MANUFACTURING PRACTICE FOR FINISHED PHARMACEUTICALS Authority 21 U S C 321 351 352 355 360b 371 374 The CGMP regulations 21 CFR parts 210 and 211 for finished pharmaceutical manufacturing do not specifically address the requirement to
what is 21 cfr 210 and 211

what is 21 cfr 210 and 211
https://www.flosum.com/hubfs/Imported_Blog_Media/1b95222c8a95a48d432b9a0932b32602a699738c-300x300.png

What s The Difference FDA 21 CFR Part 11 Vs EU Annex 11 Blue Mountain
https://www.coolblue.com/wp-content/uploads/2023/03/AdobeStock_456827650-scaled.jpeg

21 CFR Food And Drugs CGMP Finished Pharmaceuticals
https://pharmacyinfoline.com/wp-content/uploads/2022/01/21-CFR-food-and-drugs-Google-News.jpg
Regulations parts 210 and 211 and the 1978 Preamble to the CGMP regulations 2 and various comprehensive quality systems can help PART 210 CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING PROCESSING PACKING OR HOLDING OF DRUGS
211 122 Materials examination and usage criteria 211 125 Labeling issuance 211 130 Packaging and labeling operations 211 132 Tamper evident SUBCHAPTER C DRUGS GENERAL PART 210 CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING PROCESSING
More picture related to what is 21 cfr 210 and 211
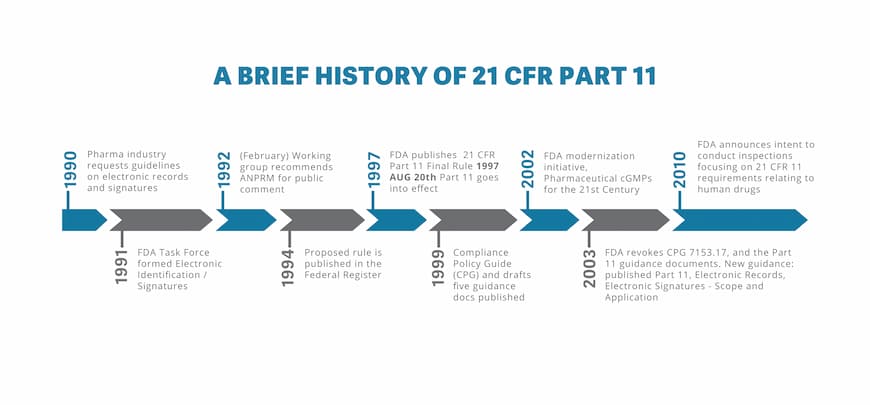
FDA 21 CFR Part 11 Explained Life Sciences ShareVault
https://www.sharevault.com/img/blog/history-21-cfr-11-timeline.jpg.pagespeed.ce.1CjgoT0NwI.jpg

Understanding 21 CFR Part 211 Gilero
https://www.gilero.com/wp-content/uploads/2021/08/21-CFR-Part-210-211-Quality-Assurance-1024x535-1.jpg

21 Cfr Part 820 Booklet My XXX Hot Girl
https://key2compliance.com/wp-content/uploads/2020/09/1360en_cfr210-211_front_2021-800.jpg
21 CFR Part 210 and 21 CFR Part 211 are the cornerstones of the FDA s cGMP regulations for the pharmaceutical industry These regulations Table of Contents Introduction to 21 CFR Part 211 guidelines for pharmaceuticals Purpose of 21 CFR Part 211 Scope of 21 CFR Part 211 Difference between 21 CFR Part 210 and
Side by Side Comparison 21 CFR Parts 110 111 211 and 820 REGULATIONS Part 110 CURRENT GOOD MANUFACTURING PRACTICE IN 21 CFR Parts 210 and 211 Regulations Document Control Subpart F Section 211 100 There shall be written procedures for production and process control

FDA 21 CFR 210 And 211 Quality Assurance By Operon Strategist Medical
https://image.isu.pub/181204064310-19ef0cca1c77ecb249e2797414d88dde/jpg/page_1.jpg

What Is 21 CFR Part 11 And Why Do You Need To Know More About It
https://hvax.in/blog/wp-content/uploads/2020/07/main-qimg-edcac00d9b01f3b6ac6af2b7af8c91b5-1024x529.png
what is 21 cfr 210 and 211 - The Electronic Code of Federal Regulations Title 21 Displaying title 21 up to date as of 2 20 2024 Title 21 was last amended 2 12 2024 view historical versions