what is 1 66054 x 10 24 Convert any regular number to scientific notation with this online tool Enter a number and get the result with the exponent of 10 and the step by step explanation
In summary The atomic mass unit u 1 660538782 10 27 kg or 10 24g and 1u 1 661x10 24g This number is achieved by dividing the mass of a nucleus by the Find the values of various physical constants used in chemistry such as Avogadro s number Boltzmann constant Faraday constant and more See the units symbols and definitions of
what is 1 66054 x 10 24
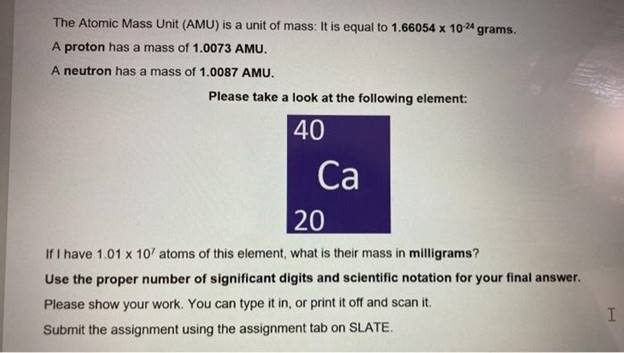
what is 1 66054 x 10 24
https://files.transtutors.com/book/qimg/997ea09b-942b-4734-a93a-06ac2fddbddb.png
Densidade E Massa Espec fica Resumo Para O Enem E Vestibulares
http://www.projetoagathaedu.com.br/imagens/blog/materias/quimica/grandezas-atomicas/carbono.png

Solved Use The Conversion Factor I Amu 1 66054 X 10 G To Chegg
https://media.cheggcdn.com/study/318/318a8c64-0825-47d3-ad17-c01bd9db9d67/image.png
Learn what an atomic mass unit AMU is how it is measured and converted and how it relates to grams per mole An AMU is equal to one twelfth the mass of a carbon 12 Answer 1 007 Explanation Identify the conversion factor given in the problem which is 1 text amu 1 66054 times 10 24 text g 1amu 1 66054 10 24g To find how many atomic
Instant Answer Step 1 4 1 Start with the given atomic weight of boron 10 811 amu Step 2 4 2 Use the conversion factor to convert amu to grams 1 amu 1 66054 x 10 24 g Step 3 4 3 Set up the conversion factor 1 amu 1 66054 10 24 g and 1 amu 6 02214 10 24 amu 3 3 2 Average Atomic Masses atomic weight average atomic mass 3 3 3 Formula and Molecular Weights formula
More picture related to what is 1 66054 x 10 24

MEDIAN Don Steward Mathematics Teaching Cuisenaire Rods And Fractions
https://1.bp.blogspot.com/-G4pb4PlI_w8/WQd5apcCJFI/AAAAAAAAVMQ/vtdmXupuPiEIMhwiKg4ziBv8X9r8fwexACLcB/s1600/Picture3.png
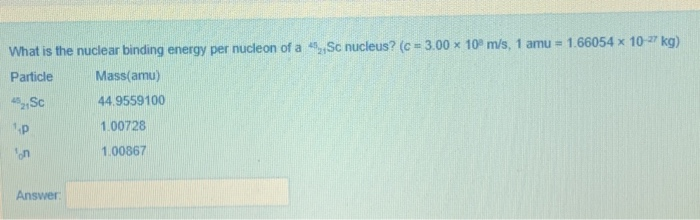
Solved What Is The Nuclear Binding Energy Per Nucleon Of A Chegg
https://media.cheggcdn.com/media/75e/75ea8b26-d61f-4d09-b17b-9b94f0e25279/image.png

6 Piece Metric Color Combo Wrench Set
https://www.harborfreight.com/media/catalog/product/i/m/image_16539.jpg
Free math problem solver answers your algebra homework questions with step by step explanations Enter any equation and get the value of x by best method possible The calculator shows step by step examples and works for one or many variables
One amu equals 1 661 10 24 g a A Mg 24 atom weighs 23 985 amu Convert this to grams and micrograms b if an atom weights 1 395 10 22 g convert this Solution Step 1 The objective is to determine the binding energy for M o 42 96 nucleus per mole and per nucleon View the full answer Step 2 Unlock Answer Unlock Previous

RTX Wheels RTX Off Road Blast II Size 16X8 17X9 18X9 Http
https://i.pinimg.com/736x/10/24/37/1024378880d9861a1408052e2b659d50--cartoon-network-truck-wheels.jpg
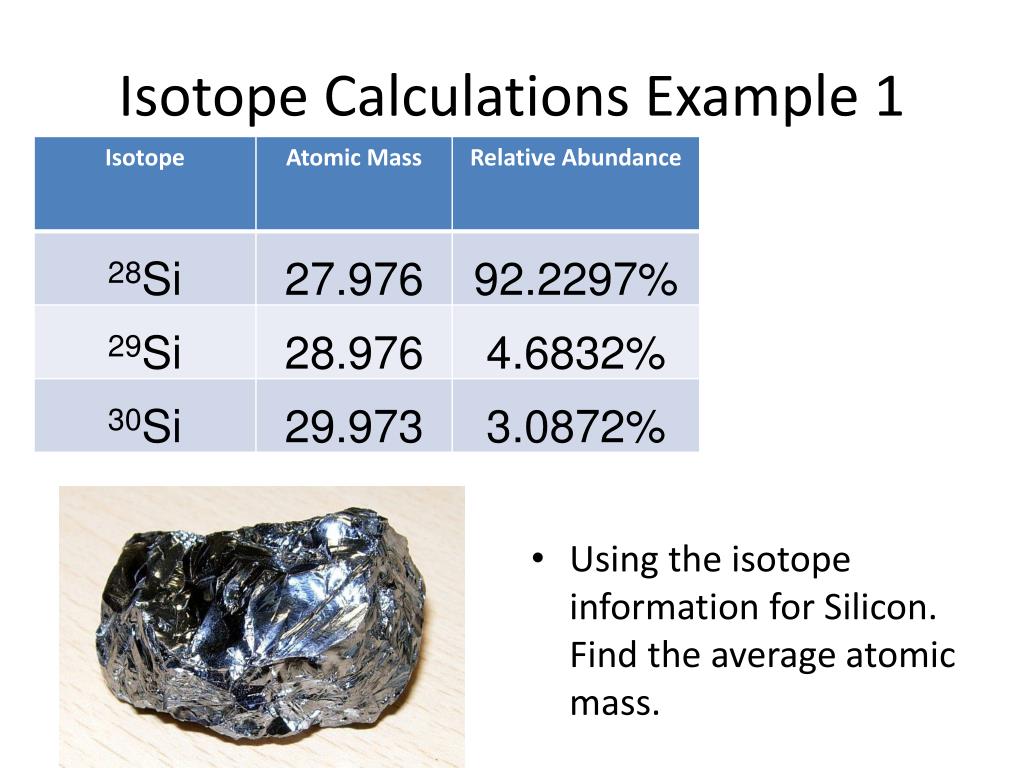
PPT Isotopes Atomic Numbers And Mass Numbers PowerPoint
https://image3.slideserve.com/6700609/isotope-calculations-example-1-l.jpg
what is 1 66054 x 10 24 - Instant Answer Step 1 4 1 Start with the given atomic weight of boron 10 811 amu Step 2 4 2 Use the conversion factor to convert amu to grams 1 amu 1 66054 x 10 24 g Step 3 4 3 Set up the conversion factor