what does m mean in quantum numbers Most conserved quantum numbers are additive so in an elementary particle reaction the sum of the quantum numbers should be the same before and after the reaction However some usually called a parity are multiplicative i e their product is conserved All multiplicative quantum numbers belong to a symmetry like parity in which applying the symmetry transformation twice is equivalent to doing nothing involution
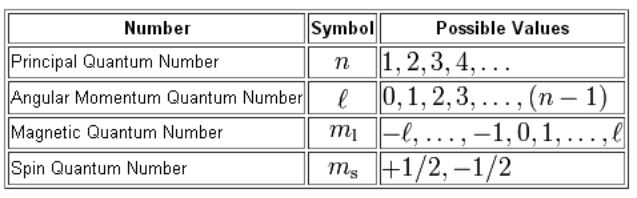
Magnetic Quantum Number m l The magnetic quantum number describes the split in the electron s energy sublevel into two or more levels It is used to project the angular momentum along a specific axis MAGNETIC QUANTUM NUMBER m l Represents the number of possible orientations in 3 D space for each type of orbital Since the type of orbital is determined by l the value of m l ranges between l and l such that m l l
what does m mean in quantum numbers
what does m mean in quantum numbers
https://cdn1.byjus.com/wp-content/uploads/2018/07/Electonic-Quantum-Numbers.jpg
How To Calculate Mode In Maths Haiper
http://i.ebayimg.com/00/s/MTA4NFgxNjAw/$(KGrHqZHJ!4E8+hp(UW,BPTSiMV(1!~~60_57.JPG
Quantum Number Easy To Understand Definition
https://gamesmartz.com/upload/subjects/science/800/quantum-number.png
Magnetic Quantum Number symbol m the orientation in space of the electron orbital A spherical orbital has only one orientation in space But others such as a dumbbell shaped orbital may have more than one In atomic physics a magnetic quantum number is a quantum number used to distinguish quantum states of an electron or other particle according to its angular momentum along a given axis in
The magnetic quantum number m describes the orientation of the orbital in space It specifies the number of orbitals within a sublevel and the spatial distribution of the electron within those The magnetic quantum number m is a quantum number that describes the orientation of an electron s orbital in a magnetic field It is an essential part of the set of quantum numbers that
More picture related to what does m mean in quantum numbers
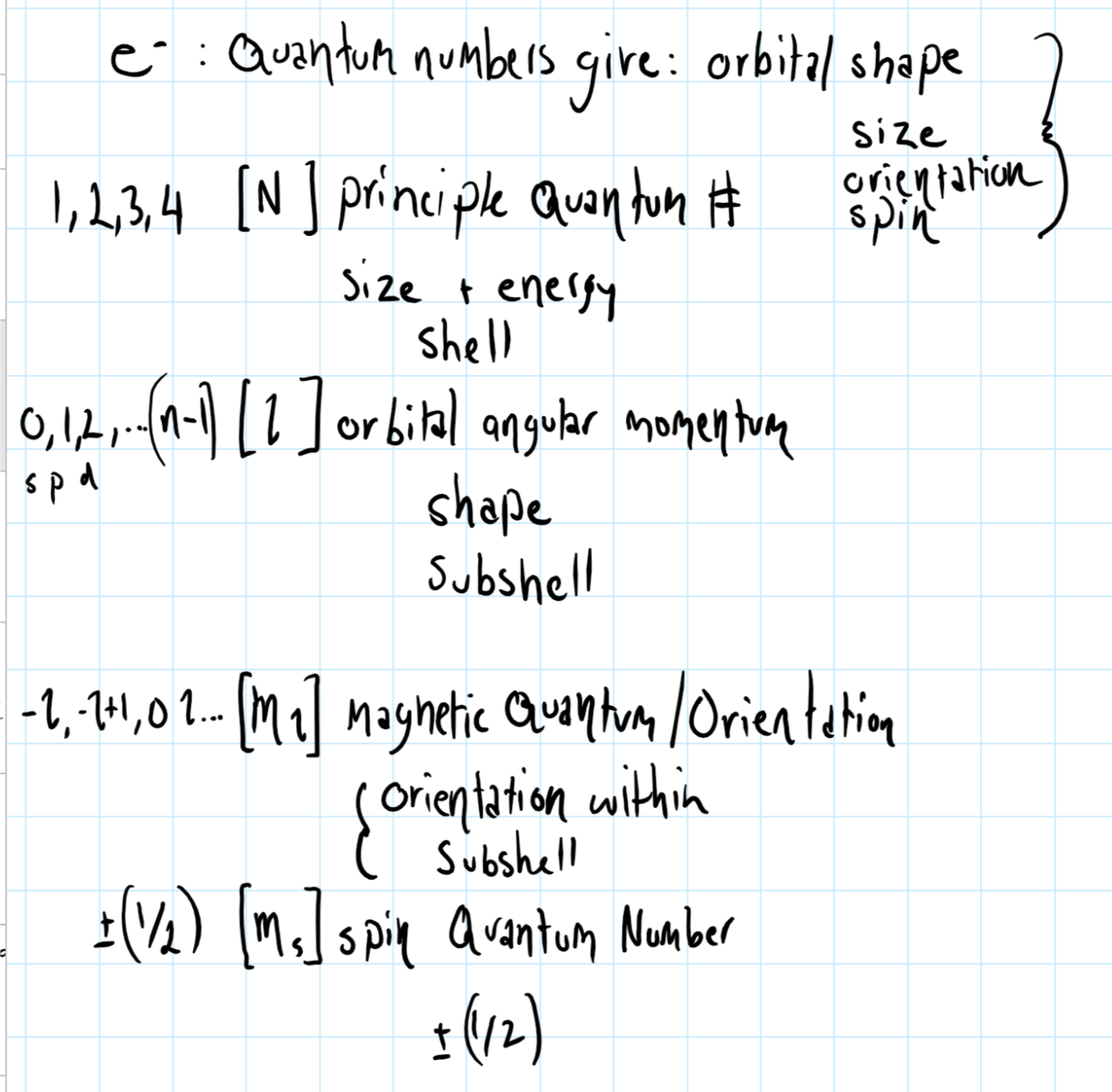
What Do The Four Quantum Numbers Describe About An Electron Socratic
https://useruploads.socratic.org/hoLToecTdOytIvUTOrAw_quantum number notation.png

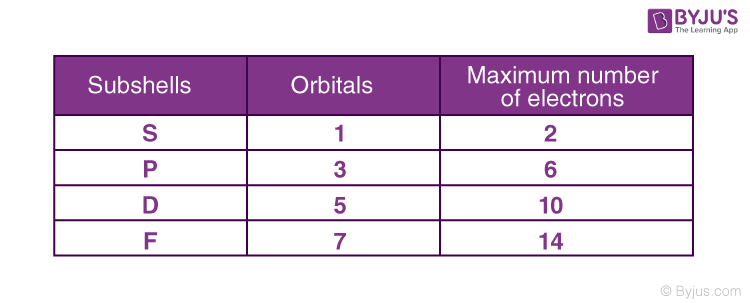
Quantum Numbers
https://thechemistrynotes.com/wp-content/uploads/2023/05/Quantum-Numbers.jpg
Magnetic Quantum Number Definition Schrodinger Equation
https://cdn1.byjus.com/wp-content/uploads/2020/09/Magnetic-Quantum-Number-1.png
Quantum mechanics uses four quantum numbers n l ml and ms to define wavefunction The first three quantum numbers provide information about the spatial Define quantum number Calculate angle of angular momentum vector with an axis Define spin quantum number Physical characteristics that are quantized such as energy charge and angular momentum are of such importance
The magnetic quantum number is denoted by the symbol ml whose values depend on the azimuthal quantum number l The formula is as follows For each value of l ml takes values Quantum numbers can be used to describe the quantum state of an electron There are four quantum numbers for atoms n 1 2 3 principal quantum number describes the
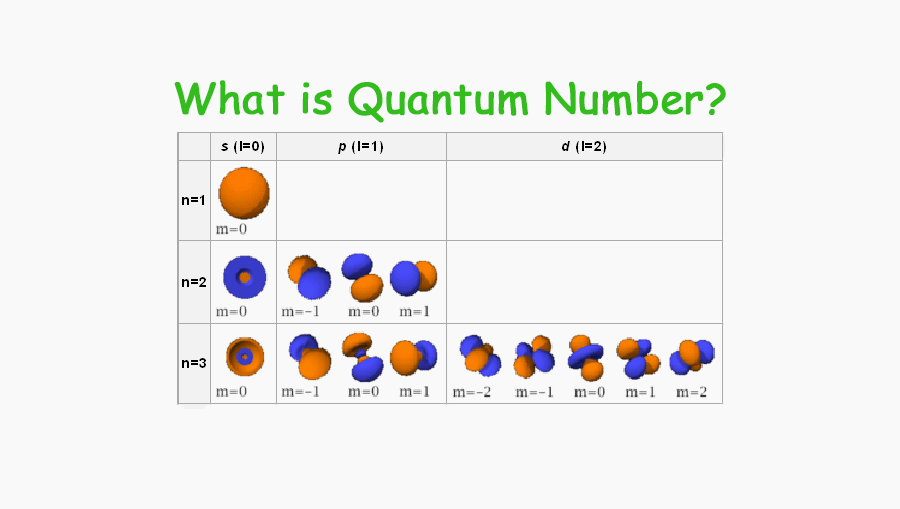
What Is Quantum Number What Do Quantum Number Determine Tuition Tube
https://tuitiontube.com/wp-content/uploads/2020/11/What-is-quantum-number.gif

Explain Different Types Of Quantum Numbers
https://i.ytimg.com/vi/wnLcJH-Heyo/sddefault.jpg
what does m mean in quantum numbers - The magnetic quantum number m describes the orientation of the orbital in space It specifies the number of orbitals within a sublevel and the spatial distribution of the electron within those