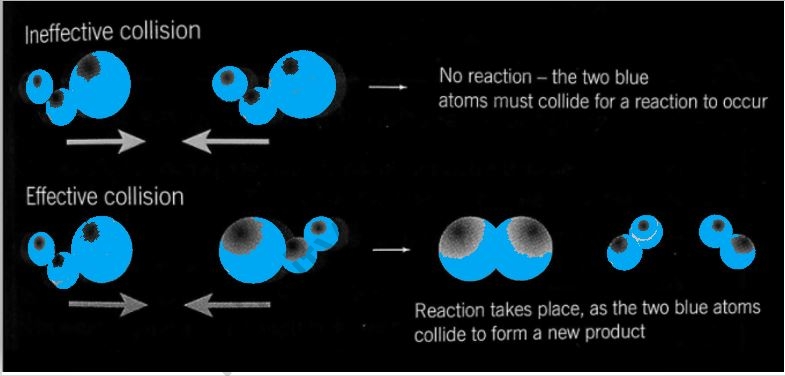
what do you mean by collision theory Collision theory explains why different reactions occur at different rates and suggests ways to change the rate of a reaction Collision theory states that for a chemical reaction to occur the reacting particles must collide with one another
Collision theory explains why different reactions occur at different rates and suggests ways to change the rate of a reaction Collision theory states that for a chemical reaction to occur the reacting particles must collide with one another Collision theory is a principle of chemistry used to predict the rates of chemical reactions It states that when suitable particles of the reactant hit each other with the correct orientation only a certain amount of collisions result in a perceptible or notable change these successful changes are called successful collisions
what do you mean by collision theory
what do you mean by collision theory
https://infinitylearn.com/surge/wp-content/uploads/2022/03/Capture-4.1-2.jpg

What Do You Mean the System Is Down It Can t Be Down We Have Work
https://technoadvantage.com/wp-content/uploads/2021/10/Lightening-stike-1080x675.jpg

What Do You Mean 360
https://p1.ssl.qhimg.com/t01493e24e0eedab308.jpg
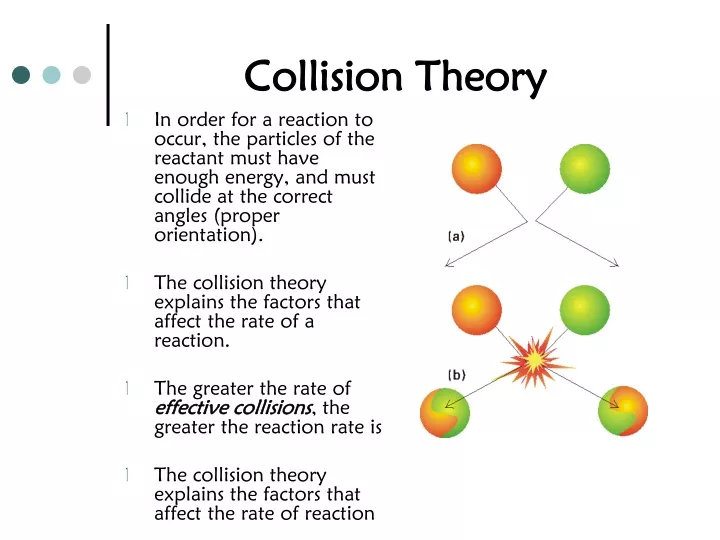
Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates The Arrhenius equation describes the relation between a reaction s rate constant and its activation energy temperature and dependence on collision orientation Collision theory Altering factors Activation energy Catalysts Potential energy diagrams Collision theory For a chemical reaction to occur the reactant molecules must collide with
The number of collisions that take place per second per unit volume of the reaction mixture is known as collision frequency To learn more about collision theory Activation energy Arrhenius equation with Videos and FAQs Visit BYJU s for more content About Transcript Collision theory states that molecules must collide to react For most reactions however only a small fraction of collisions produce a reaction
More picture related to what do you mean by collision theory

Collision Theory Gizmo Name
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/048386471e5eff4bfc71e9c83f60dd79/thumb_1200_1553.png

Question Video Naming The Theory Describing How Chemical Reactions
https://media.nagwa.com/138140727892/en/thumbnail_l.jpeg
PPT Collision Theory PowerPoint Presentation Free Download ID 9507407
https://cdn5.slideserve.com/9507407/collision-theory-n.jpg
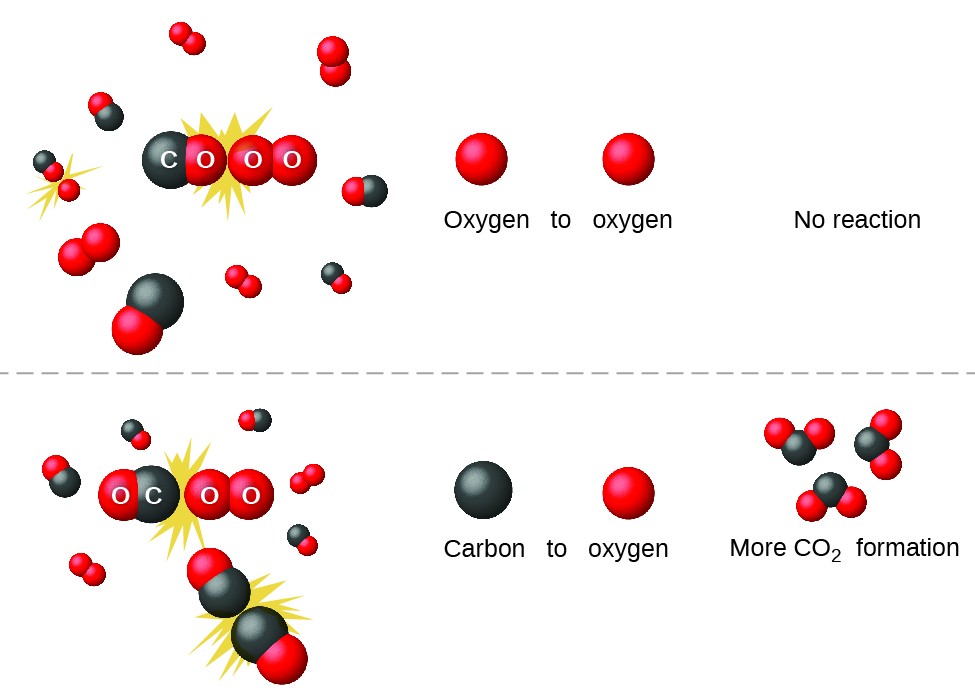
Collision theory explains why most reaction rates increase as concentrations increase With an increase in the concentration of any reacting substance the chances for collisions between molecules are increased because there are more molecules per unit of volume Collision theory states that in order for a reaction to occur reactant particles must collide with enough kinetic energy to overcome the activation energy barrier
This page describes the collision theory of reaction rates It concentrates on the key things which decide whether a particular collision will result in a reaction in particular the energy of the collision and whether or not the molecules hit each other the right way around the orientation of the collision Collision theory states that for a reaction to take place reactants must collide properly The rate of reaction is equal to the frequency of collisions Collision theory is limited to gases because frequencies of atomic collisions can only be
What Do You Mean By Endowment Policy PolicyBachat
https://www.policybachat.com/FAQsImages/what-do-you-mean-by-endowment-policy-14708.jpg
Collision Theory Chemistry For Majors Atoms First Course Hero
https://assets.coursehero.com/study-guides/lumen/images/chemistryatomsfirst/collision-theory/CNX_Chem_12_05_COandO21.jpg
what do you mean by collision theory - The number of collisions that take place per second per unit volume of the reaction mixture is known as collision frequency To learn more about collision theory Activation energy Arrhenius equation with Videos and FAQs Visit BYJU s for more content