ppm to mol l gas To convert ppm to molarity or molarity to ppm you only need to know one thing the molar mass of the dissolved element or molecule If you take molarity with units text mol text L mol L and multiply it by the molar mass with units text g text mol g mol you get text g text L g L
One part per million by volume is equal to a volume of a given gas mixed in a million volumes of air A micro liter volume of gas in one liter of air would therefore be equal to 1 ppm Today s more and more there is an interest to express gas concentrations in metric units i e g m 3 Although expressing gaseous concentrations in g m 3 Conversion from mass concentration to ppm By dividing the mass of gas in a cubic meter by the molar mass of gas we can get the number of moles of gas in a cubic meter Multiplying the number of moles of gas by the molar volume of gas for a given temperature and pressure we get the volume of gas in liters per cubic meter of total
ppm to mol l gas
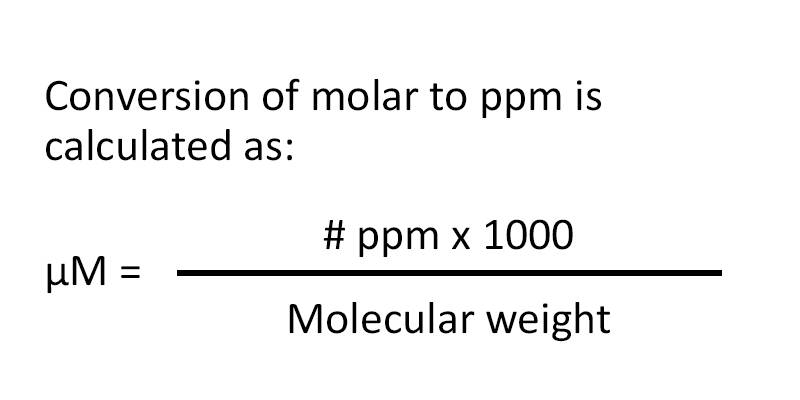
ppm to mol l gas
https://propg.ifas.ufl.edu/images/01-biology/01-hormones/hormonesmolarity/image3.jpg
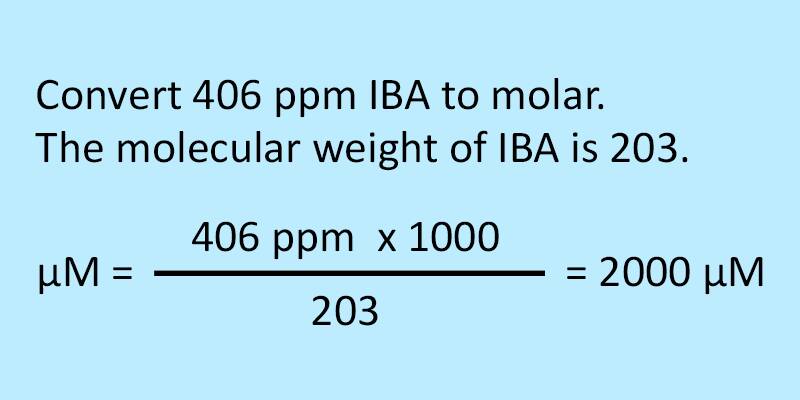
Plant Hormones Molarity
https://propg.ifas.ufl.edu/images/01-biology/01-hormones/hormonesmolarity/image4.jpg

How To Calculate Ppm With Molarity Beyondlalaf
https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/117/11708086.png
How to convert PPM to molarity First determine the PPM Calculate the total parts per million of the solution or substance Next determine the molar mass Measure or calculate the molar mass in g mol Finally calculate the molarity Calculate the molarity with the equation above Conversions Between Moles and Gas Volume Molar volume at STP can be used to convert from moles to gas volume and from gas volume to moles The equality of 1mol 22 4L 1 mol 22 4 L is the basis for the conversion factor Example 10 7 1 10 7 1 Converting Gas Volume to Moles
Ppm parts per million This unit that expresses concentration in parts per million is measured as the volume denoted in litres L of a substance found in 1L of a medium such as air mg m 3 milligram per cubic metre Compute answers using Wolfram s breakthrough technology knowledgebase relied on by millions of students professionals For math science nutrition history
More picture related to ppm to mol l gas

How To Calculate Ppm By Mass Brewdase
https://www.researchgate.net/profile/Azad-Karimi-2/post/How_to_convert_ppm_to_weight_part_per_hundred_in_case_of_Major_element/attachment/5fbfe0885a4aa800016ca093/AS:962169009098771%401606410376658/image/Screenshot_2020-11-26+Molecular+Weights+and+Conversion+Factors.png

How To Calculate Ppm Of Iron Using Tha Mass Value Caqweadventures
https://i.ytimg.com/vi/_VPA7irczjc/maxresdefault.jpg

How To Calculate Ppm To Concentration Daseae
https://i.ytimg.com/vi/mrLVjmPqfRg/maxresdefault.jpg
How To Convert PPM to Molarity YouTube The Organic Chemistry Tutor 7 77M subscribers Subscribed 1 7K 185K views 3 years ago New AP General Chemistry Video Playlist This chemistry Ppm 1 000 000 c s 106 c s 1 where c molar mass volume or mass of the solute component mole m3 ft3 kg lbm s molar mass volume or mass of the solution mole m3 ft3 kg lbm In the metric system ppm for mass can be expressed in terms of milligram versus kg where 1 mg kg 1 part per million
C ppm C mg kg 10 6 c mol L M g mol 998 2071 kg m 3 1000 c mol L M g mol How to convert ppm to Hz The frequency variation in hertz Hz is equal to the frequency stability FS in ppm times the frequency in hertz Hz divided by 1000000 f Hz FS ppm f Hz 1000000 Example A mixture of gases isn t any different and to know the weight of the gas you want you just need to multiply the molar mass of the gas with the proportion in the mixture ppm is the proportion here So each ppm of methane will contribute about 16 1 000 000 g to each 22 4L of the gas mixture
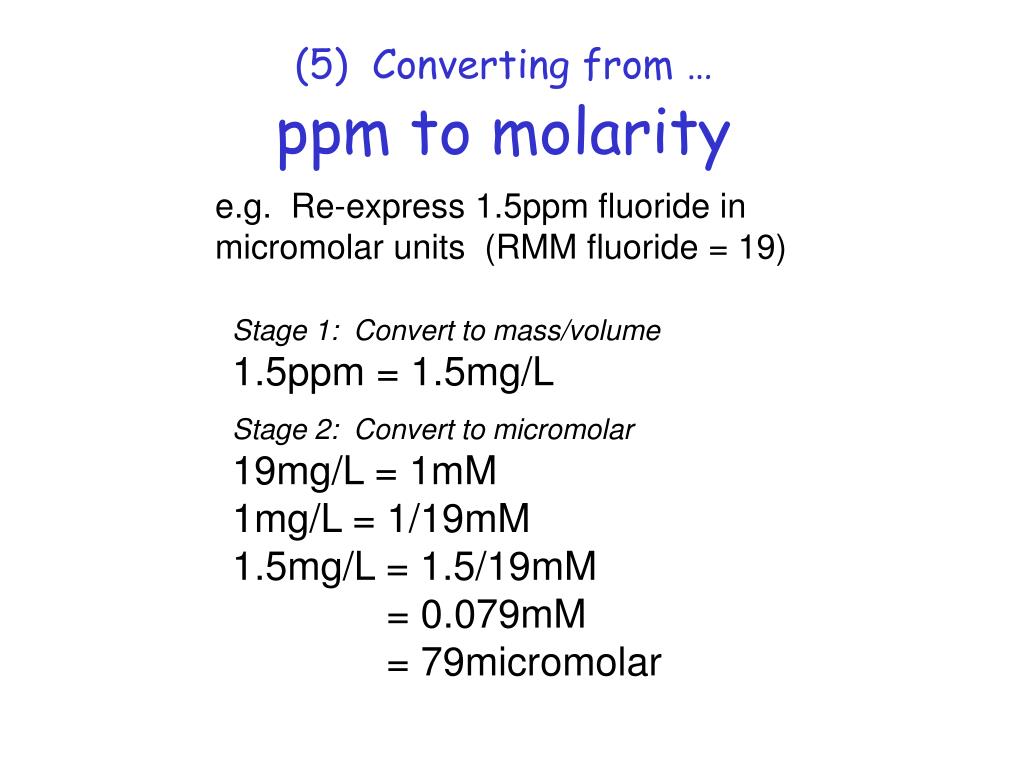
How To Calculate Ppm Powerpoint Mangojawer
https://image.slideserve.com/885306/5-converting-from-ppm-to-molarity-l.jpg

How To Calculate Ppm Of Solution Portalnaxre
http://drcalef.com/Chem160/Chapter13/img16.jpg
ppm to mol l gas - Molar volume L Concentration g m 3 or ng L Concentration ppb x Molecular mass g mol Molar volume L For more information on calculating atmospheric concentrations refer to Application Note 025 Note that the ppm and ppb concentrations in these formulae are by volume and the molar volume at 1 atm and 25 C is 24 45 L