oxygen g mol mass Oxygen O2 CID 977 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information
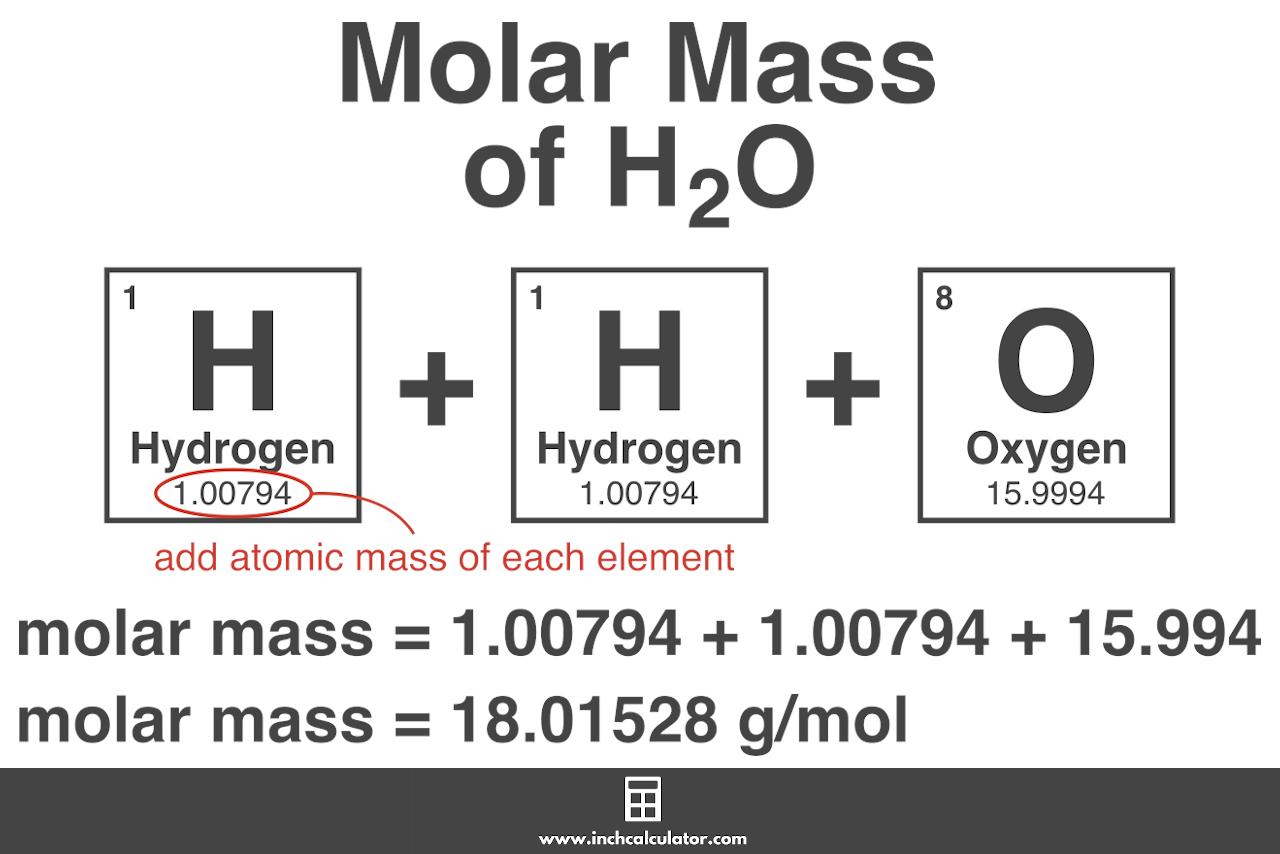
Generalizing this definition the molar mass of any substance in grams per mole is numerically equal to the mass of that substance expressed in atomic mass units For example The molar mass of Oxygen is 15 999 g mol 1 Now to calculate the molar mass of O2 you just have to add the molar mass of all the individual atoms that are present in O2
oxygen g mol mass
oxygen g mol mass
https://www.inchcalculator.com/wp-content/uploads/2021/07/molar-mass.png

How Many Moles In A Gram Of Oxygen Slide Share
https://i.ytimg.com/vi/m7FbbQYhqhk/maxresdefault.jpg

Question Video Calculating The Moles Of A Reactant Consumed In A
https://media.nagwa.com/854174618957/en/thumbnail_l.jpeg
Oxygen is a chemical element with symbol O and atomic number 8 Oxygen was discovered by Carl Wilhelm Scheele in Uppsala in 1773 Type the number of Oxygen O you want to What is the molar mass of oxygen O2 The molar mass of molecular oxygen O2 is approximately 32 00 g mol The molar mass of molecular oxygen O2 is calculated as
As you calculated in Example 1 the molecular mass of ethanol is 46 069 amu Because 1 mol of ethanol contains 2 mol of carbon atoms 2 12 011 g 6 mol of hydrogen Molar mass of O2 31 9988 g mol Convert grams O2 to moles or moles O2 to grams Molecular weight calculation 15 9994 2 Percent composition by element Element Oxygen
More picture related to oxygen g mol mass

A Sample Of A Compound That Contains Only The Elements C H And N Is
https://us-static.z-dn.net/files/d93/60e9bccf495bb376d4a2359a412d7e22.jpg

Solved A 9 780 g Gaseous Mixture Contains Ethane C2H6 And Chegg
https://media.cheggcdn.com/media/0eb/0ebfb215-59c5-4c4d-a7ae-ee066bf54141/phpZ2h4Cq.png
Solved A Compound With A Molar Mass Of 78 0 G mol Is Found To Contain
https://www.coursehero.com/qa/attachment/19823691/
Element Oxygen O Group 16 Atomic Number 8 p block Mass 15 999 Sources facts uses scarcity SRI podcasts alchemical symbols videos and images Oxygen gas is a diatomic molecule O 2 First look up the atomic mass atomic weight of the element which is 16 00 Next multiply this value by 2 the subscript following the symbol for oxygen O The molar mass of O 2 is
The Molar Mass Of O2 Is 32 0 G Mol What Mass In Grams Of O2 Must React To Form 3 80 Mol Of Al2O3 To calculate the mass of O2 required to form 3 80 mol of Al2O3 we need to use Find molar masses of carbon 12 01 g mol and oxygen 16 g mol Multiply the molar mass of each element by the number of atoms in the compound s formula then add
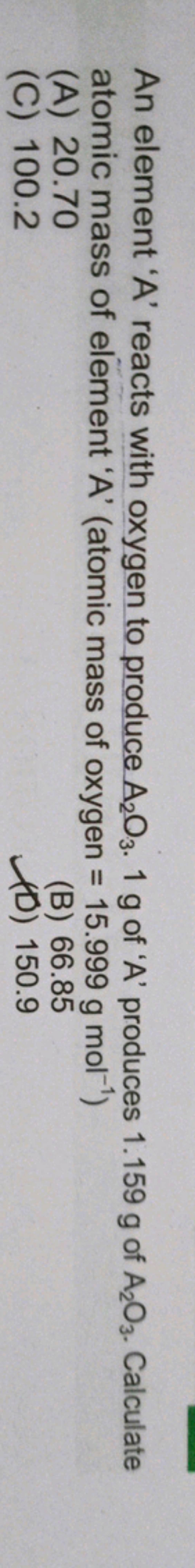
Atomic Mass Of Element A atomic Mass Of Oxygen 15 999 G Mol 1
https://static-images.findfilo.com/classroom/1683309949911_gorqlyoa_3971235.jpg
/chemical-periodic-table-of-elements-with-color-cells-vector-illustration-840464844-ff61af51081b4fb1959504105e232e01.jpg)
Periodic Table With Molar Mass Mainride
https://www.thoughtco.com/thmb/HbLPtq-NdGb7NF_F9OOmFfVjRBw=/5444x3062/smart/filters:no_upscale()/chemical-periodic-table-of-elements-with-color-cells-vector-illustration-840464844-ff61af51081b4fb1959504105e232e01.jpg
oxygen g mol mass - We know that the oxygen gas we breathe is the diatomic molecule O2 in fact most common elemental gases are diatomic what are the exceptions to this generalization