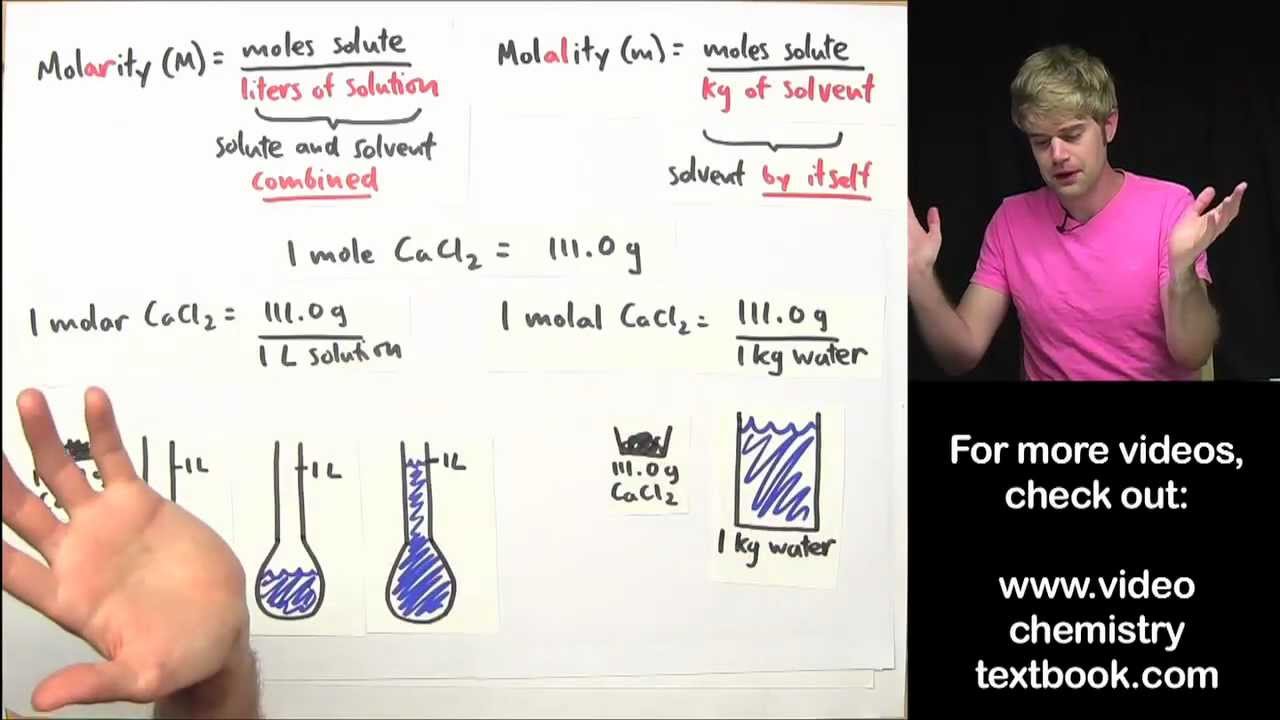
moles vs molarity Learn how molarity and molality differ The molality of a solution is equal to the moles of solute divided by the mass of solvent in kilograms while the molarity of a solution is equal to the
Molarity and molality are both measures of the concentration of a chemical solution Molarity is the ratio of moles to volume of the solution mol L while molality is the ratio of moles to the mass of the solvent mol kg Learn the difference between molarity and molality two common measurements of solution concentration in chemistry Molarity is the number of moles per volume of
moles vs molarity
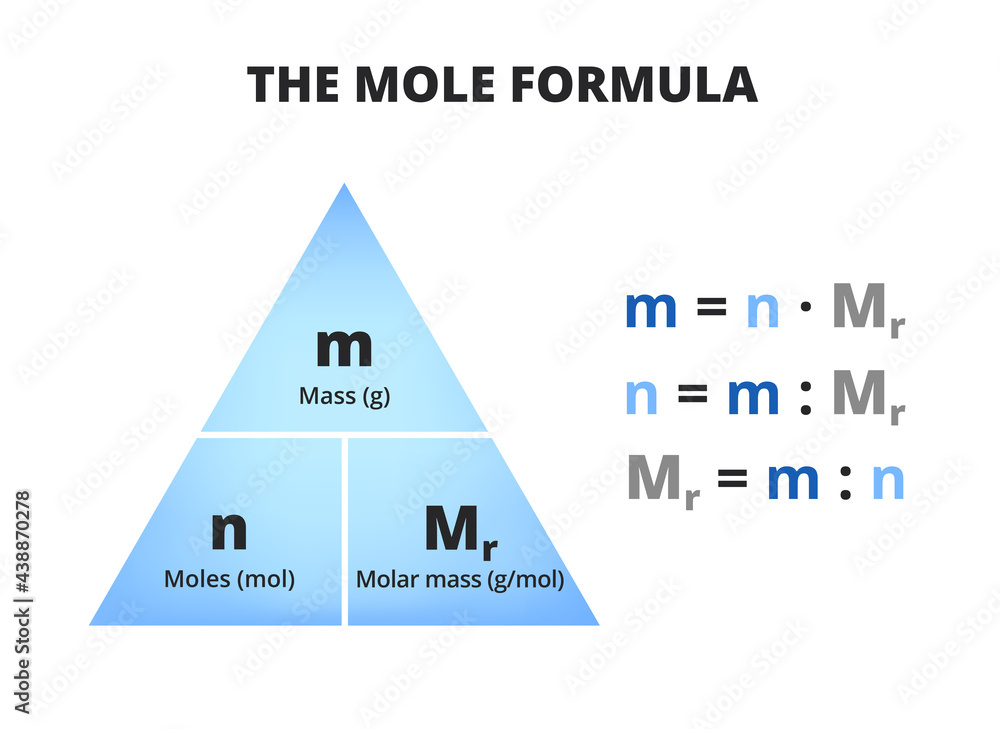
moles vs molarity
https://as1.ftcdn.net/v2/jpg/04/38/87/02/1000_F_438870278_cpb2rdHX7RZQFdU6fA3u5lZ9SdXHBh9e.jpg
Solubility Curves Lessons Blendspace
https://i.ytimg.com/vi/96oNrVnTk50/maxresdefault.jpg
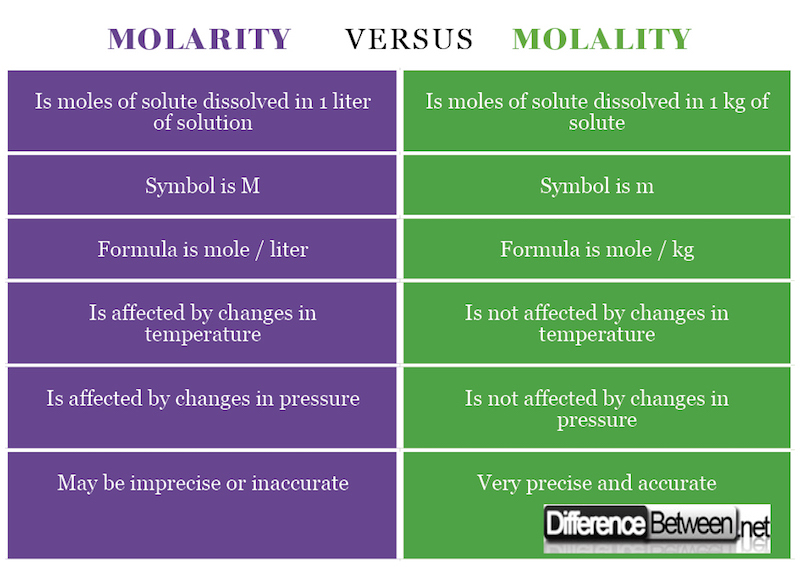
Difference Between Molarity And Molality Difference Between
http://www.differencebetween.net/wp-content/uploads/2018/02/Molarity-VERSUS-Molality-.jpg
Molarity is the number of moles of a substance per litre of solution also known as molar concentration A capital M signifies solutions labelled with molar concentration A 1 0 M solution contains 1 mole of solvent per litre of solution Molarity is the ratio of the moles of a solute to the total liters of a solution The solution includes both the solute and the solvent Molality on the other hand is the ratio of the moles of a solute to the kilograms of a solvent
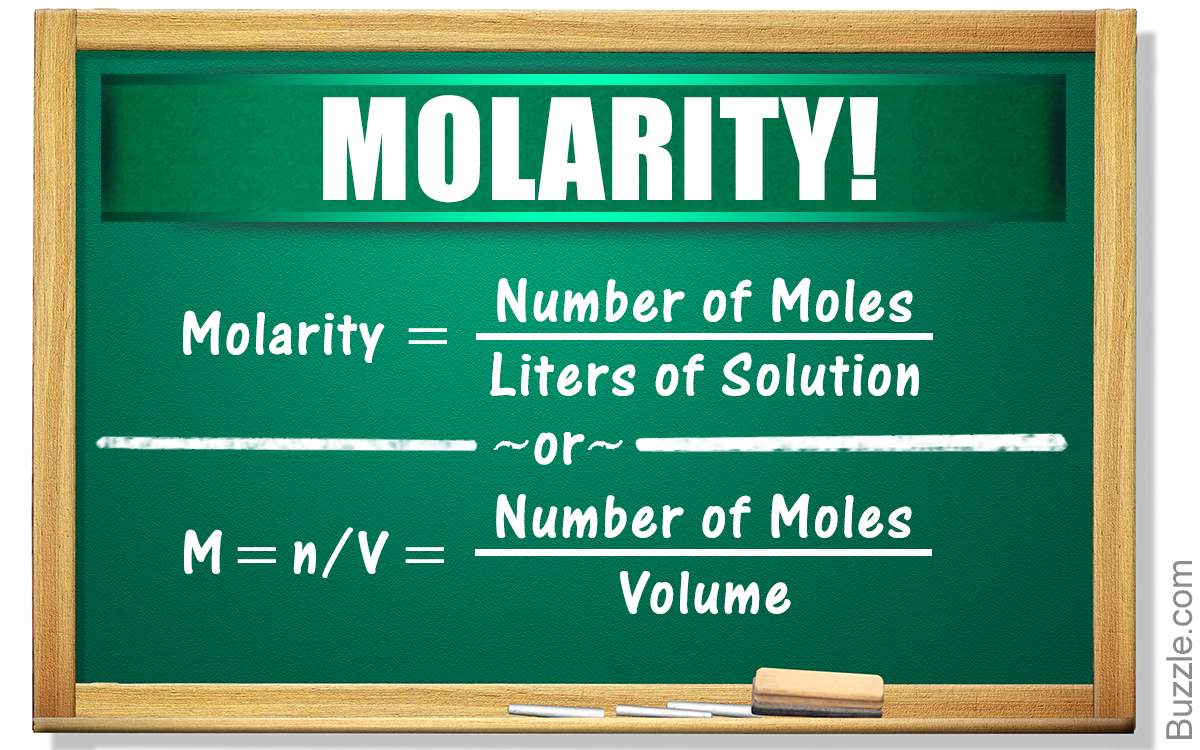
The most common way to express solution concentration is molarity M which is defined as the amount of solute in moles divided by the volume of solution in liters M moles of solute liters Learn how to calculate molarity the number of moles of solute per liter of solution and other units of concentration See examples formulas and diagrams of solution
More picture related to moles vs molarity
Spice Of Lyfe Hardest Chemical Equation To Balance
https://lh3.googleusercontent.com/proxy/cUmRfqCMUB6E28ei10YDPsm_CfMlLPs2q8vQ8LMKC7XXj1J8DEEVRCyqzL9yiy6grQlSa_42IgXdSR6qde3w1mNvEeJsIruMfRnRcWj0EexPhiLNGA=s0-d

Moles Molarity And Concentration Edexcel 9 1 Separate Triple Science
https://d1e4pidl3fu268.cloudfront.net/29d72ef0-640f-495a-aaa6-c9d620eb44a1/Molarity.jpg

Molarity Vs Molality
https://image.slidesharecdn.com/molarityvsmolality-160819164859/95/molarity-vs-molality-2-638.jpg?cb=1471625443
Molarity allows us to express the amount of a solute in a given volume of solution while molality considers the amount of solute in relation to the mass of the solvent Differentiating between molarity and molality is crucial in Molality is calculated by dividing the moles of solute by the mass of the solvent in kilograms On the other hand molarity represented by the symbol M is a measure of the concentration of
In chemistry the most commonly used unit for molarity is the number of moles per liter having the unit symbol mol L or mol dm 3 in SI units A solution with a concentration of 1 mol L is For example calculate the molarity of a 795 milliliter solution that is prepared by dissolving 1 64 moles of gold III chloride in water In order to calculate the molarity of a

Calculating Molarity Solving For Moles Grams 4 Practice Examples
https://i.ytimg.com/vi/9VlA_kVcFi0/maxresdefault.jpg
How To Calculate Osmolarity From Molarity
https://ibiologia.com/wp-content/uploads/2019/05/1200-350623-172868395.jpg
moles vs molarity - Learn how to calculate molarity the number of moles of solute per liter of solution and other units of concentration See examples formulas and diagrams of solution