molecular and molar mass For an element the molar mass is the mass of 1 mol of atoms of that element for a covalent molecular compound it is the mass of 1 mol of molecules of that compound for an
In chemistry the molar mass M sometimes called molecular weight or formula weight but see related quantities for usage of a chemical compound is defined as the ratio between the mass and the amount of substance measured in moles of any sample of the compound The molar mass is a bulk not molecular property of a substance The molar mass is an average of many instances of th For an element the molar mass is the mass of 1 mol of atoms of that element for a covalent molecular compound it is the mass of 1 mol of molecules of that compound for an ionic compound it is the mass of 1 mol of formula units
molecular and molar mass
molecular and molar mass
https://psiberg.com/wp-content/uploads/2022/03/Atomic-Mass-vs-Molar-Mass-Difference-table.svg

Difference Between Molar Mass And Molecular Mass Difference Between
http://www.differencebetween.net/wp-content/uploads/2018/03/Molar-Mass-VERSUS-Molecular-Mass-.jpg

Difference Between Molar Mass And Molecular Mass Compare The
https://i2.wp.com/www.differencebetween.com/wp-content/uploads/2011/08/Difference-Between-Molar-Mass-and-Molecular-Mass-Tabular-Form.jpg?w=734&ssl=1
Molar mass measures the mass of a mole of a substance Molecular mass measures the mass of molecules Molar mass is measured in terms of g mol or kg mol The mass of one mole of atoms molecules ions is called its molar mass M expressed in g mol Numerically the molar mass is equal to the atomic mass of a given atom or a molecule so we can look up the molar mass of an element
When we weigh one mole of a substance on a balance this is called a molar mass and has the units g mol grams per mole This idea is very critical in chemistry because it is used all the Molar mass The molar mass of a substance is the mass of one mole The amount of substance that contains the same number of particles as there are atoms in 12 g of carbon 12 contains
More picture related to molecular and molar mass
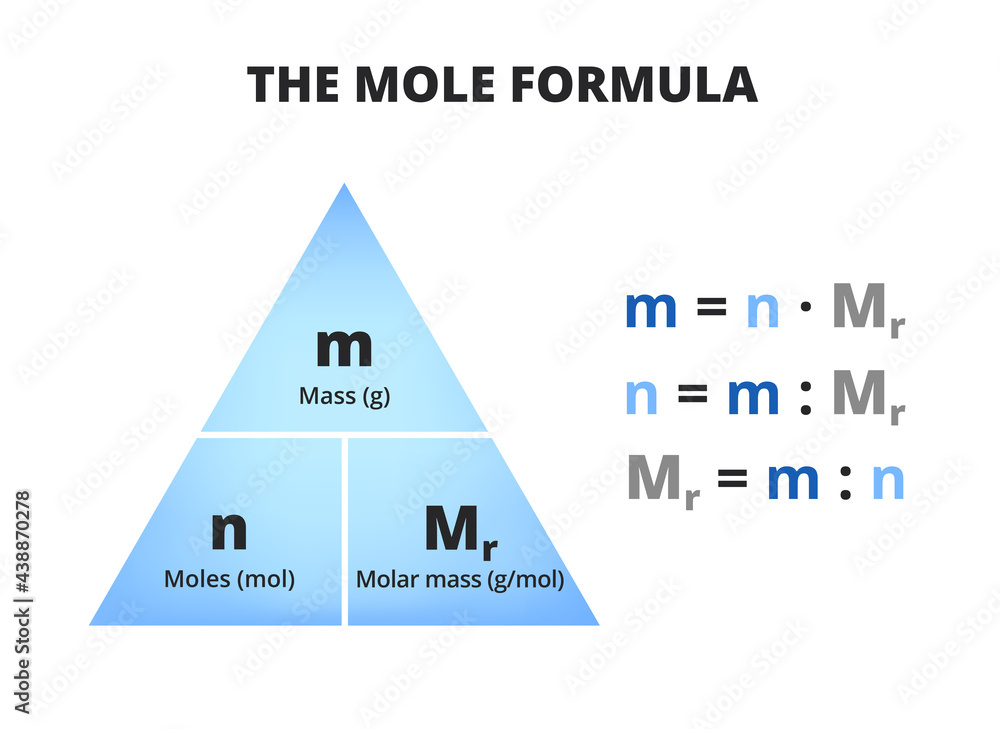
Moles Triangle
https://as1.ftcdn.net/v2/jpg/04/38/87/02/1000_F_438870278_cpb2rdHX7RZQFdU6fA3u5lZ9SdXHBh9e.jpg

What Are The Similarities In How Formula Weight And Molar Mass Are
https://us-static.z-dn.net/files/d85/2cb8d049a475d557c95c10786e530567.jpg
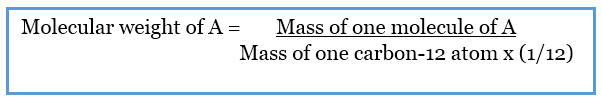
Difference Between Molar Mass And Molecular Weight Definition
http://pediaa.com/wp-content/uploads/2017/06/Difference-Between-Molar-Mass-and-Molecular-Weight-3.png
The composition of a compound may be determined from its chemical formula and the atomic masses of the elements that make up the compound The mass of a molecule is called its molecular mass The Molecular weight is the mass of a molecule of a substance It can also be called molecular mass The units for molecular weight are atomic mass units amu Molar mass is the mass of one mole of a substance Molar mass
This molecular weight calculator can determine the molecular weight or molar mass of a molecule based on its formula and also provides the percentage of the atoms The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol The molar mass of a compound can be calculated by adding
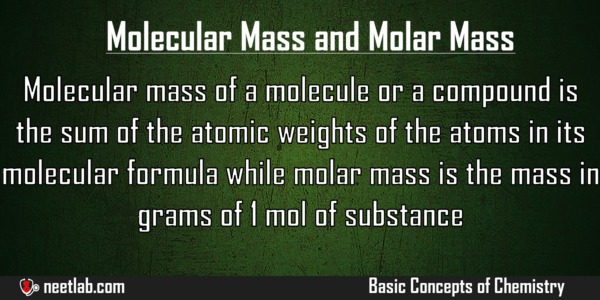
Difference Between Molecular Mass And Molar Mass NEETLab
https://neetlab.com/wp-content/uploads/2017/11/Difference-between-Molecular-Mass-and-Molar-Mass-Basic-Concepts-of-Chemistry-Explanation-.jpg
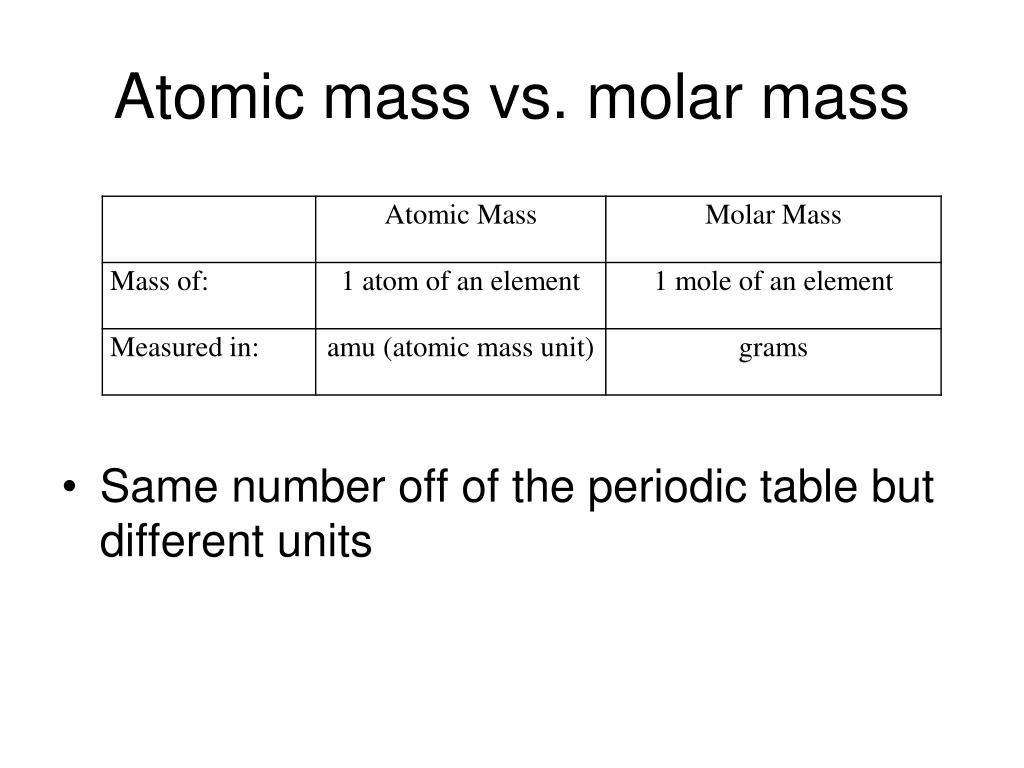
PPT Atoms The Building Blocks Of Matter PowerPoint Presentation
https://image3.slideserve.com/6384515/atomic-mass-vs-molar-mass-l.jpg
molecular and molar mass - When we weigh one mole of a substance on a balance this is called a molar mass and has the units g mol grams per mole This idea is very critical in chemistry because it is used all the