mole fraction to ppm Mol fraction Mass fraction Volume fraction Molar concentration moles per volume Mass per volume sometimes called ppm Parts per million mol Parts per million weight Parts per million volume Mol Fraction multiply by 100 for mass mole or volume Mass Fraction multiply by 100 for mass mole or volume Volume Fraction
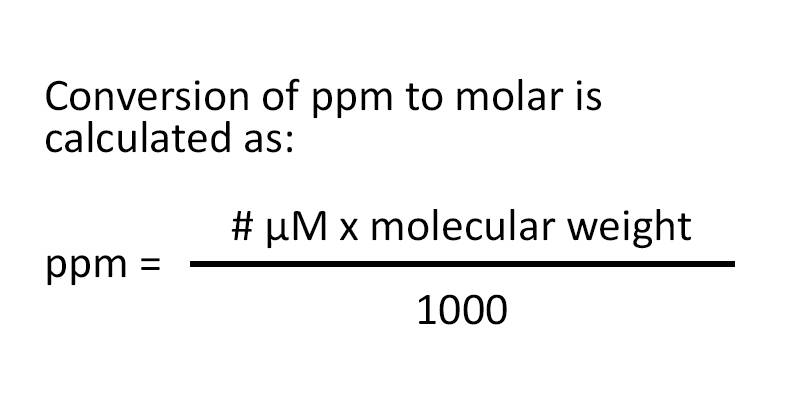
Ppm moles L m mol 1000 footnotesize text ppm frac text moles text L cdot m text mol cdot 1000 ppm L moles m mol 1000 Example 1 Seawater vs Drinking water The average salt content in seawater is equivalent to 0 599 M 0 599 text M 0 599 M NaCl text NaCl NaCl although sea salts are not entirely made up July 30 2023 by GEGCalculators Molarity to ppm Calculator Convert to ppm ppm to Molarity Calculator ppm Convert to Molarity FAQs How do you convert molarity to ppm To convert molarity M to ppm parts per million you can use the following formula ppm molarity molar mass 10 6 What is 0 1 M in ppm
mole fraction to ppm

mole fraction to ppm
https://i.ytimg.com/vi/Qr0QQcg0s1M/maxresdefault.jpg

How To Calculate Ppm From Mole Fraction Darelobrooklyn
https://i.ytimg.com/vi/HkfH330Fs-Y/maxresdefault.jpg
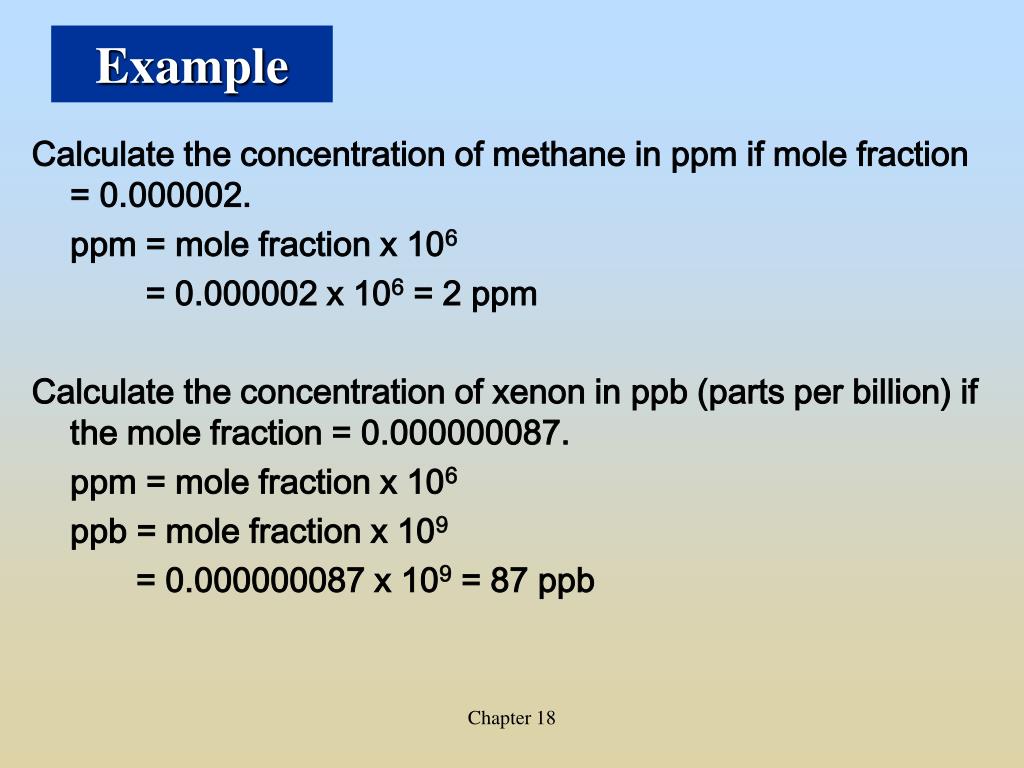
PPT Chapter 18 Chemistry Of The Environment PowerPoint Presentation
https://image3.slideserve.com/6908529/example-l.jpg
How to convert ppm to decimal fraction The part P in decimal is equal to the part P in ppm divided by 1000000 P decimal P ppm 1000000 Example Find the decimal fraction of 300ppm P decimal 300ppm 1000000 0 0003 How to convert decimal fraction to ppm The part P in ppm is equal to the part P in decimal times 1000000 P ppm Mole Fraction One way to express relative amounts of substances in a mixture is with the mole fraction Mole fraction X is the ratio of moles of one substance in a mixture to the total number of moles of all substances For a mixture of two substances ce A and ce B the mole fractions of each would be written as follows
PPMs or parts per million are a unit of measurement of concentration A part per million corresponds to a concentration of one part of a substance per 999 999 parts of solvent out of a total of one million PPM are scientific units used to compare quantities with the same measurement units moles square meters Ppm is defined as ppm 1 000 000 c s 10 6 c s 1 where c molar mass volume or mass of the solute component mole m 3 ft 3 kg lb m s molar mass volume or mass of the solution mole m 3 ft 3 kg lb m In the metric system ppm for mass can be expressed in terms of milligram versus kg where 1 mg kg 1 part per million
More picture related to mole fraction to ppm

Solutions Class03 Mole Fraction Molality W W Ppm YouTube
https://i.ytimg.com/vi/Yck3MbffkkI/maxresdefault.jpg
Plant Hormones Molarity
http://propg.ifas.ufl.edu/images/01-biology/01-hormones/hormonesmolarity/image1.jpg

Parts Per Million PPM And Mole Fraction YouTube
https://i.ytimg.com/vi/g8mLZ1mgc18/maxresdefault.jpg
Molarity calculations Concentration terms Calculating one concentration term using the others Science Physical Chemistry Essentials Class 11 Some basic Concepts of Chemistry Concentration terms Mole fraction its relation with concentration terms Let s explore mole fraction its relation with concentration terms Select the appropriate unit options and then enter your number in any field of this PPM calculator and get the converted value Parts Per Notation The parts per notation is a set of pseudo units to describe small values of various dimensionless quantities e g mole fraction or mass fraction
[desc-10] [desc-11]

Units Of Concentration
https://chem.fsu.edu/chemlab/chm1046course/molefraction.gif

Mole Mass Fraction ppm Part 3 Solutions YouTube
https://i.ytimg.com/vi/i_mdD5yFf-M/maxresdefault.jpg
mole fraction to ppm - Mole Fraction One way to express relative amounts of substances in a mixture is with the mole fraction Mole fraction X is the ratio of moles of one substance in a mixture to the total number of moles of all substances For a mixture of two substances ce A and ce B the mole fractions of each would be written as follows