mole and molarity Calculate the molarity of a solution As stated previously chemists have defined several types of concentrations which each use a different chemically acceptable unit or
The most common unit of concentration is molarity which is also the most useful for calculations involving the stoichiometry of reactions in solution The molarity M is defined This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating grams per ml to moles You can also calculate the mass of a substance needed to
mole and molarity
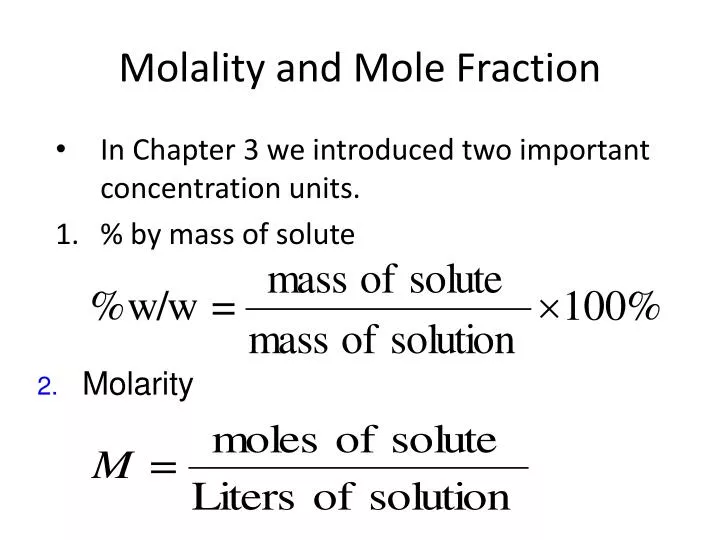
mole and molarity
https://image3.slideserve.com/6594493/molality-and-mole-fraction-n.jpg
How To Convert Mole Fraction Into Molarity And Molality Quora
https://qph.fs.quoracdn.net/main-qimg-c91794e0f6439e09c823ae0cd7690e0c

Relationship Between Molality And Molarity Mole Concept By TUC By
https://i.ytimg.com/vi/pWDVz7ZxInw/maxresdefault.jpg
Molar concentration also called molarity amount concentration or substance concentration is a measure of the concentration of a chemical species in particular of a solute in a solution in The molar mass of a substance is defined as the mass of 1 mol of that substance expressed in grams per mole and is equal to the mass of 6 022 10 23 atoms molecules or
Molarity is the number of moles of solute per liter of solution For example if you dissolve table salt in water salt is the solute and water is the solution One mole of sodium chloride weighs 58 44 grams Molarity also known as the molar concentration of a solution is the technique of calculating the amount of substance a particular chemical solution contains It is measured by considering two
More picture related to mole and molarity
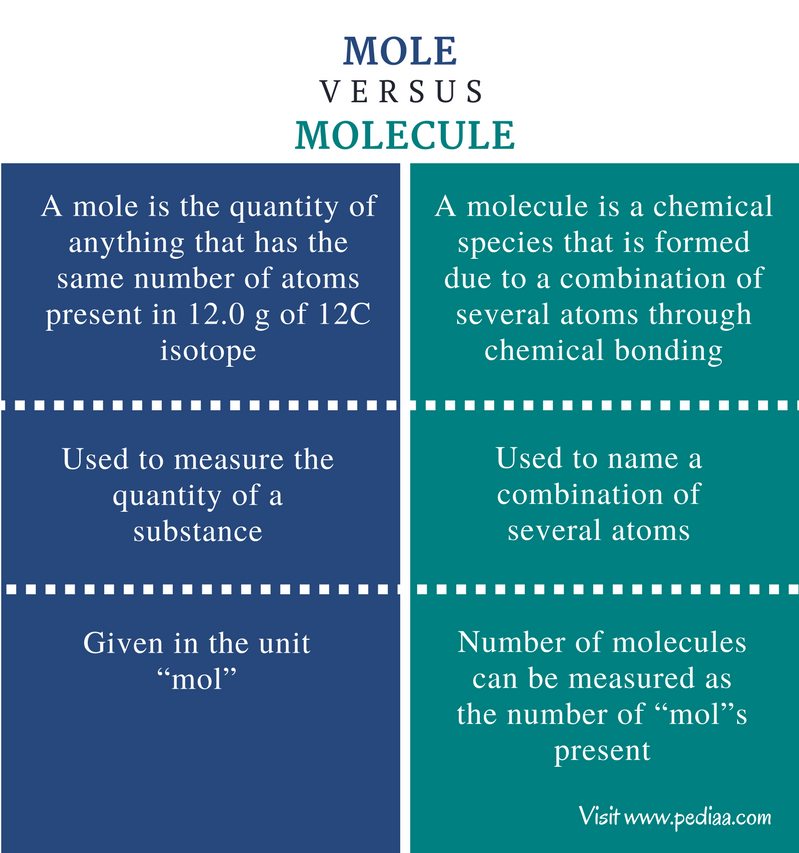
Difference Between Mole And Molecule Definition Applications Examples
http://pediaa.com/wp-content/uploads/2017/08/Difference-Between-Mole-and-Molecule-Comparison-Summary.png

Molarity Formula Science Struck
https://pixfeeds.com/images/41/374397/1280-374397-molarity-formula.png
/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png)
Learn How To Calculate Molarity Of A Solution
https://www.thoughtco.com/thmb/lEI5hXvJPRQPclvm3qcZlY9bywY=/3000x2000/filters:fill(auto,1)/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png
Molarity M is the most common way of expressing the solution concentration you will see in this chapter It expresses the concentration of a solution as the number of moles of solute in a liter The formula mass of a covalent compound is also called the molecular mass A convenient amount unit for expressing very large numbers of atoms or molecules is the mole
Yes there is a difference Molarity is the most common concentration measurement and denoted by the capital letter M Molarity is the number of moles of Molarity and molality are both measures of the concentration of a chemical solution Molarity is the ratio of moles to volume of the solution mol L while molality is the

Chemistryteacher001 s Shop Teaching Resources TES
https://d1e4pidl3fu268.cloudfront.net/f012e218-cb66-4206-999d-f13a37cabcf0/L9MoleCalculations.gif
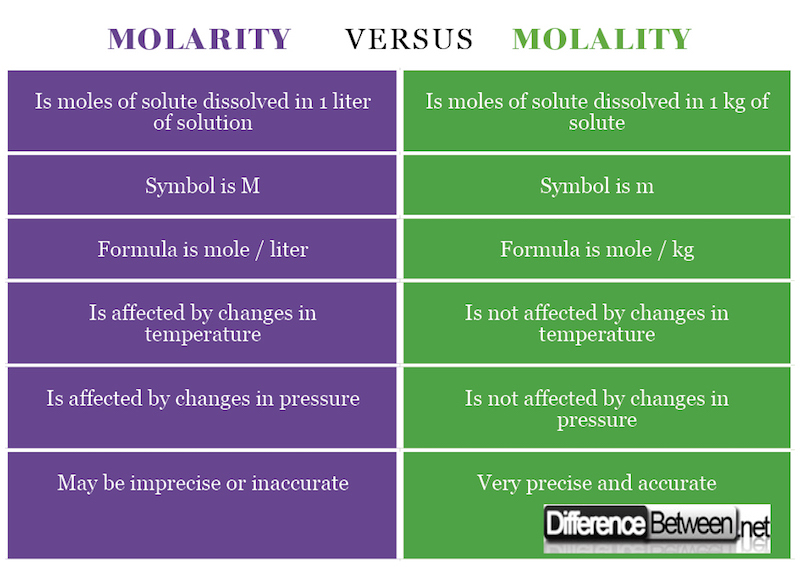
Difference Between Molarity And Molality Difference Between
http://www.differencebetween.net/wp-content/uploads/2018/02/Molarity-VERSUS-Molality-.jpg
mole and molarity - Molar concentration also called molarity amount concentration or substance concentration is a measure of the concentration of a chemical species in particular of a solute in a solution in