molar to moles formula The molecular weight is the mass of one mole of a substance typically expressed in grams per mole g mol Based on this definition the formula to calculate molarity when molecular weight
Learning Objectives Perform conversions between mass and moles of a substance Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction Use a balanced chemical Molarity to Moles Formula To calculate moles from molarity you can use the formula n M V where n Number of moles mol M Molarity M or mol L V Volume of solution L How to convert molarity to moles To convert
molar to moles formula
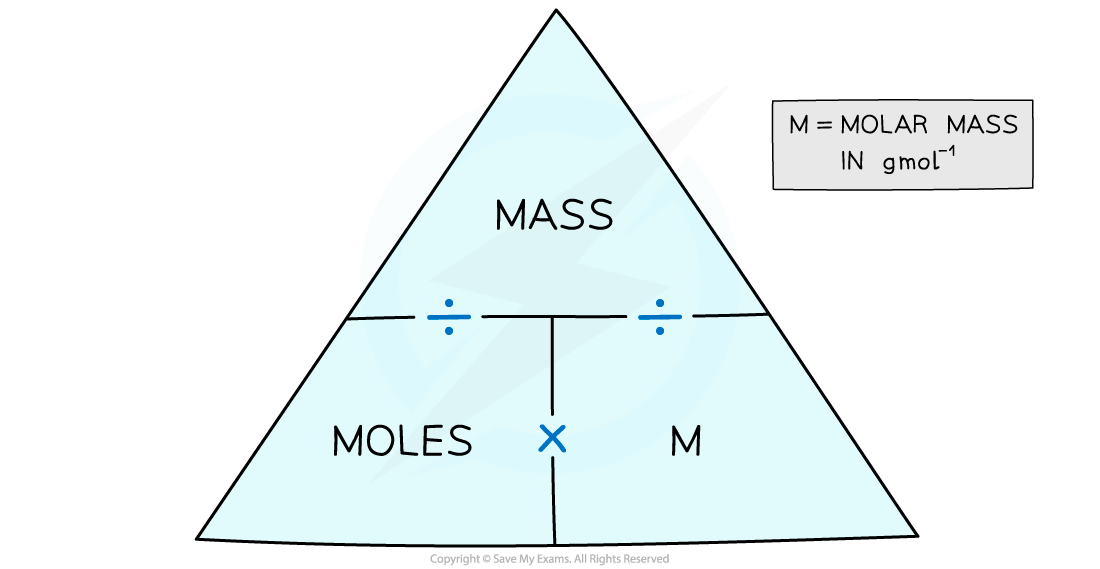
molar to moles formula
https://oss.linstitute.net/wechatimg/2022/07/1.1.5-The-Moles-Mass-Formula-Triangle-3.png
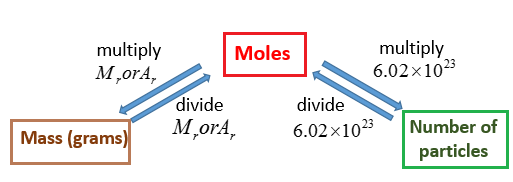
Molar Mass Grams Moles solutions Examples Activities Experiment
https://www.onlinemathlearning.com/image-files/xmole-mass-avogadro.png.pagespeed.ic.QfUP0vcyUg.png

5 Gmol Images Stock Photos Vectors Shutterstock
https://www.shutterstock.com/shutterstock/photos/1990476140/display_1500/stock-vector-the-mole-formula-triangle-or-pyramid-isolated-on-a-white-background-relationship-between-moles-1990476140.jpg
How many moles of oxygen react with hydrogen to produce 27 6 mol of ce H2O Find a balanced equation that describes the reaction Unbalanced H 2 O 2 H 2 O Balanced 2H The molar mass of a substance is the sum of the average molar masses of the atoms that compose the substance The molar mass of a substance can be used as a conversion factor
The volume L of a solution is equal to the mass g divided by the molar mass of the substance g mol divided by the molarity mol Calculate the molarity of a solution with our molar concentration calculator Plus learn the molarity To find the number of moles of atoms we use the formula Moles mass of substance relative atomic mass If we take a mole of ionic lattice or molecules of a material the mass will be the
More picture related to molar to moles formula
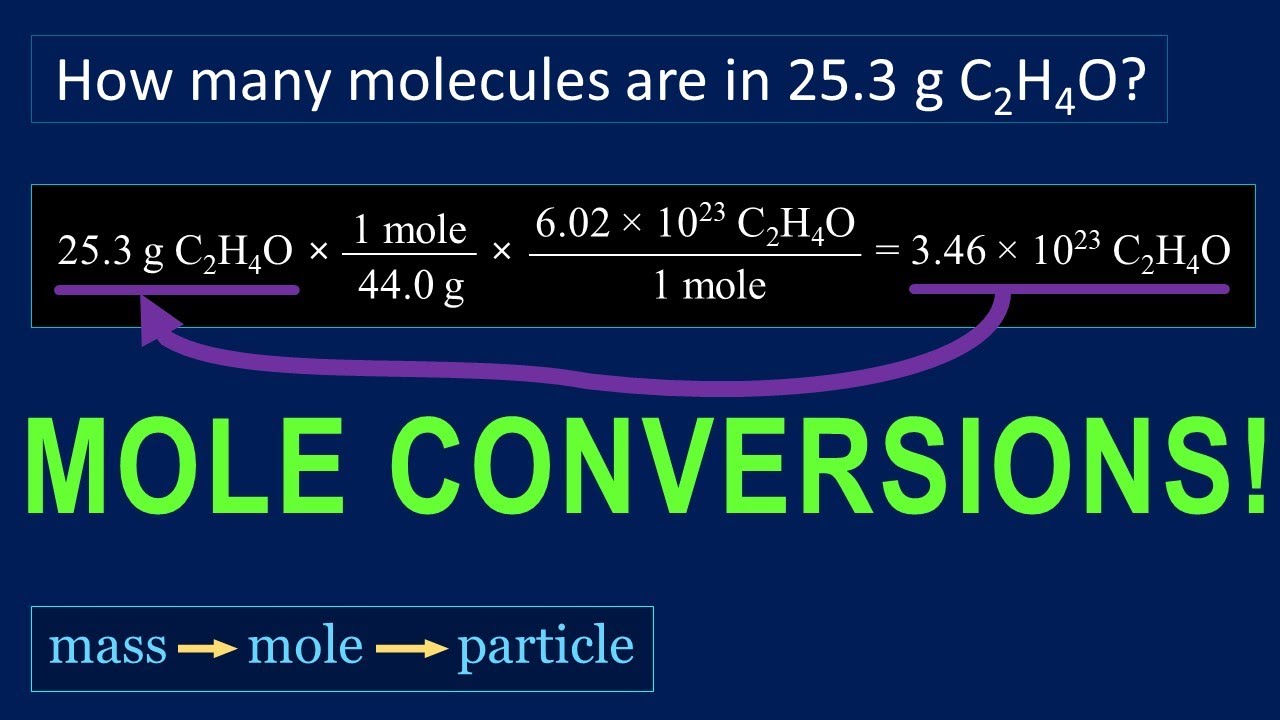
How To Convert Moles To Molecules Slideshare
https://i.ytimg.com/vi/t1pjGbwqt9o/maxresdefault.jpg

The Mole Ratio How To Calculate It Modeladvisor
https://imgmidel.modeladvisor.com/how_do_you_find_the_mole_ratio_from_the_empirical_formula.jpg

Moles To Atoms Conversion Chemistry YouTube
https://i.ytimg.com/vi/mpHQZ1PrkUI/maxresdefault.jpg
Molarity is the number of moles of a substance in one litre of solution The official symbol for molarity is c concentration but many people use the old symbol M M n V Examples for elements and compounds are given in the table below along with its formula relative formula mass RFM and the mass of 1 mole
Molarity is the number of moles of a substance in one litre of solution color blue bar ul color white a a M n Vcolor white a a where n is the number Molarity Equation As shown below the molarity of a solution is defined as the ratio of the molar amount of solute that is present in a solution relative to the volume of the
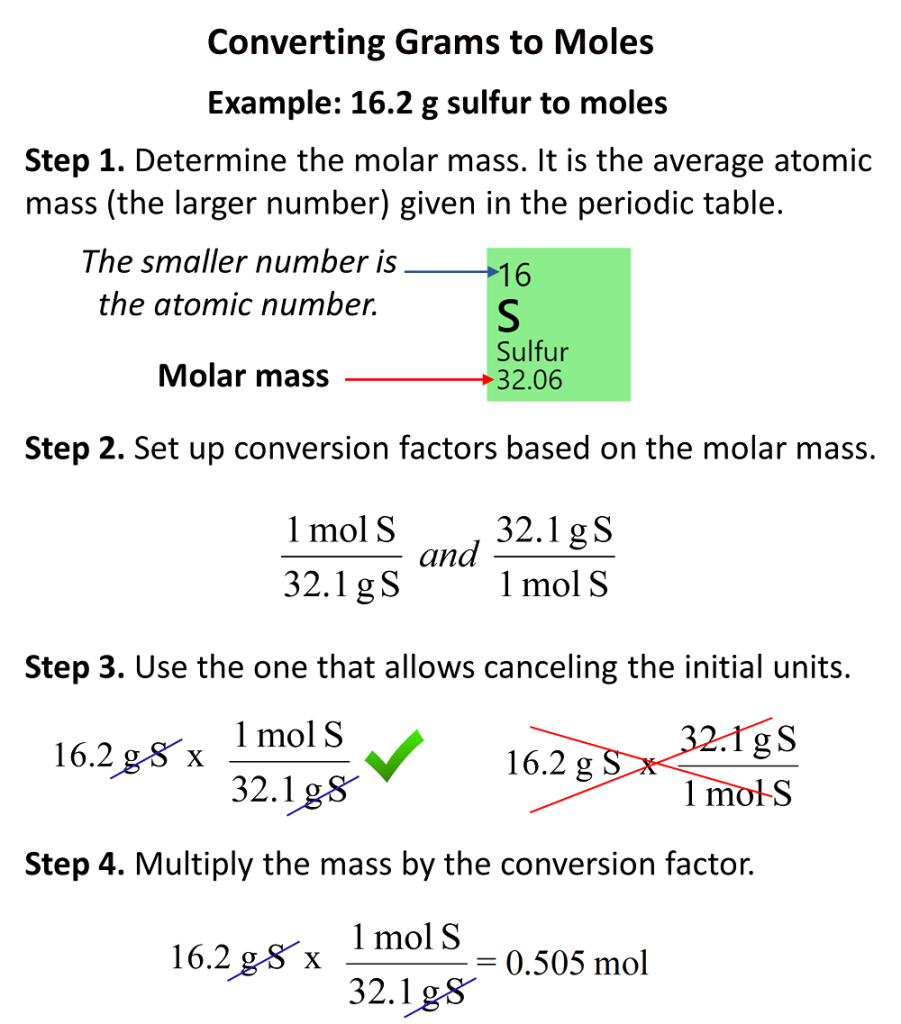
How To Convert Grams To Moles Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/12/Converting-grams-to-moles-easy-sumarry-steps-901x1024.png

Molecular HydroCarbons Mole Conversions
http://4.bp.blogspot.com/-jg6XwY8pZB0/TsyHNjAXH9I/AAAAAAAAARI/QdhD96KHtHA/s640/Mole+map.png
molar to moles formula - The volume L of a solution is equal to the mass g divided by the molar mass of the substance g mol divided by the molarity mol Calculate the molarity of a solution with our molar concentration calculator Plus learn the molarity