kg steam to m3 water I estimate that the mass defect for water molecules bound in the liquid phase is probably as large as 10 parts in 10 12 So boiling a kilogram of water generates a kilogram plus about ten nanogram of steam
Calculate online thermodynamic and transport properties of water and steam based on industrial IAPWS IF97 or scientific IAPWS 95 formulation Mollier diagrams included According the table 720 94 kJ is required to raise 1 kg of water from 0 oC to saturation temperature 170 oC The heat energy enthalpy of evaporation needed at 7 bar g to vaporize the water to steam is actually less than
kg steam to m3 water
kg steam to m3 water
https://media.cheggcdn.com/media/3fc/3fcc31f5-aaa5-4286-9389-2cb193974015/phpQO2LqZ

Density Of Water Kg m3
https://amazingconverter.com/images/page/what-is-the-density-of-water-in-kg-m3.jpg
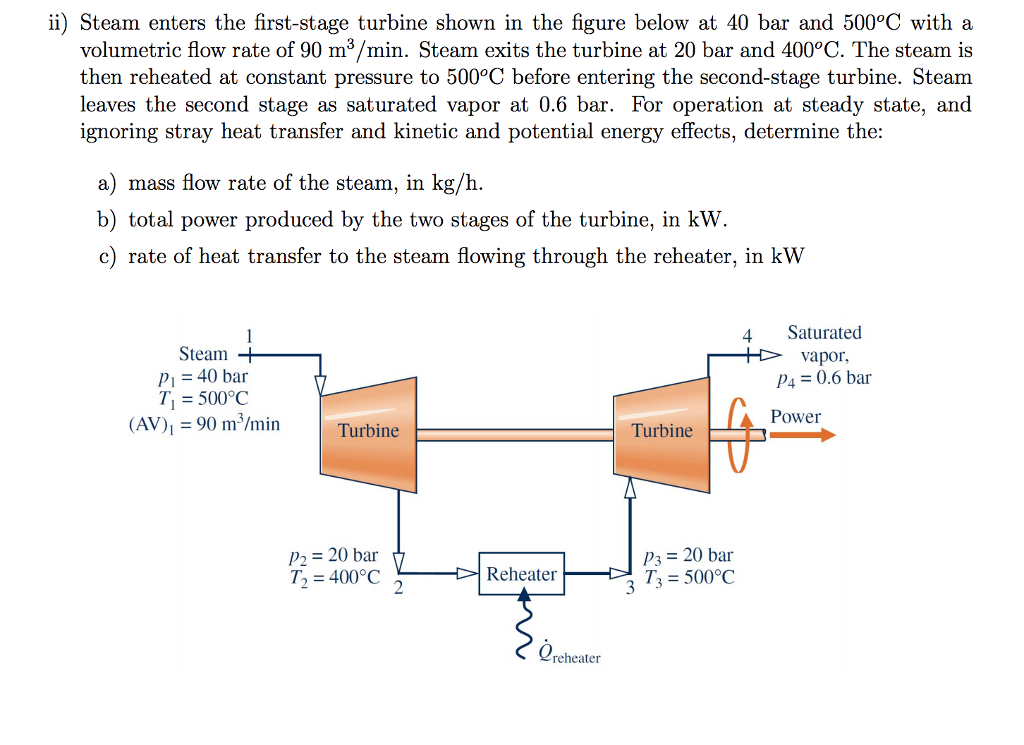
Solved Ii Steam Enters The First stage Turbine Shown In The Chegg
https://media.cheggcdn.com/media/3c3/3c3e05bb-62d5-4a9f-8440-757b9a6546ea/phpgAia3x.png
Online calculator with Saturated Steam Table by Pressure Includes 53 different calculations Equations displayed for easy reference Steam Tables Online Calculator completely free Calculate propierties of wet saturated and superheated steam steam quality and more Thermodynamics calculator based on
39 rowsSteam at boiling point properties Pressure bar c p kJ kgK kg m3 10 6 Pa s 10 6 m2 s 0 0 0 0061 1 864 0 0048 8 04 1675 0 Water Flue gases Steam Calculators All calculators Pipe diameter Steam is converted back into water through the process of condensation This involves cooling the steam until it reaches its dew point at which point it turns back into liquid
More picture related to kg steam to m3 water
Solved A Rigid Closed Tank Of Volume 3 M3 Contains 5 Kg Of Wet Steam
https://www.coursehero.com/qa/attachment/33745102/
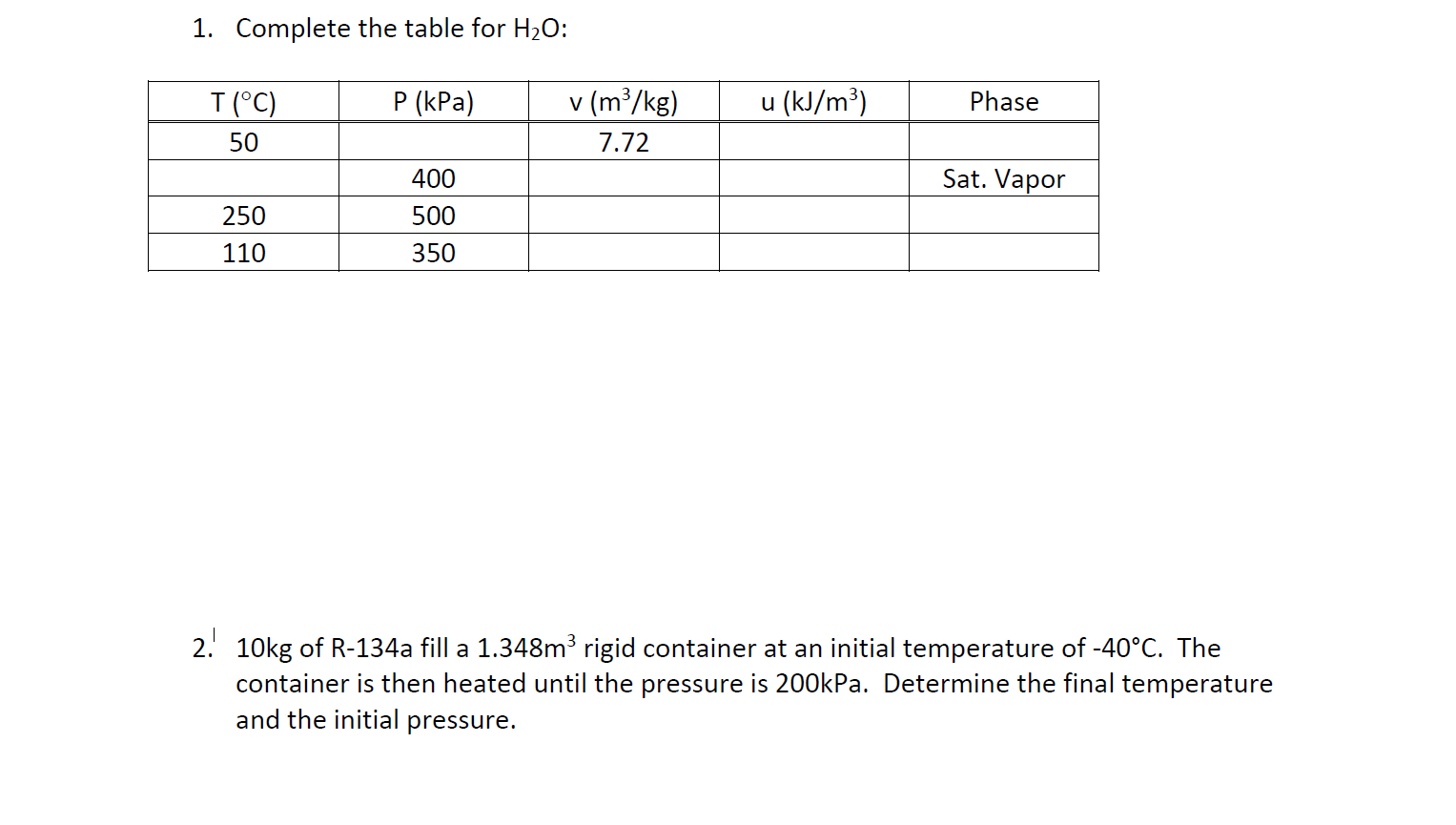
Solved 1 Complete The Table For H2O P kPa U kJ m3 Chegg
https://media.cheggcdn.com/media/d00/d00d072e-ab4a-40d1-8493-55623b75843e/phpBHPfdP.png

Example Problem 3 Determining The Quantity Of Heat Needed To Convert
https://i.ytimg.com/vi/7uuMV2nK1fQ/maxresdefault.jpg
419 kJ of energy is required to heat 1 kg of water from 0 o C to the saturation temperature 100 o C Therefore at 0 bar gauge absolute 101 33 kN m 2 and 100 o C the specific enthalpy of water is 419 kJ kg Another 2257 kJ of How to calculate steam requirements for flow and non flow applications Including warm up heat losses and running loads The optimum design for a steam system will largely depend on
The answer is 1000 We assume you are converting between kilogram water and cubic metre You can view more details on each measurement unit kilo gram or cubic metre The SI derived 1 kg of water at 100 C is converted into steam at 100 C by boiling at atmospheric pressure The volume of water changes from 1 00 10 3 m3 as a liquid to 1 671 m3 as

Termo Q a Chapter 3 Chapter 3 1 A 1 m3 Rigid Tank Contains Steam At
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/f47dbabcc8ad1994cb4a6a93178623ca/thumb_1200_1698.png
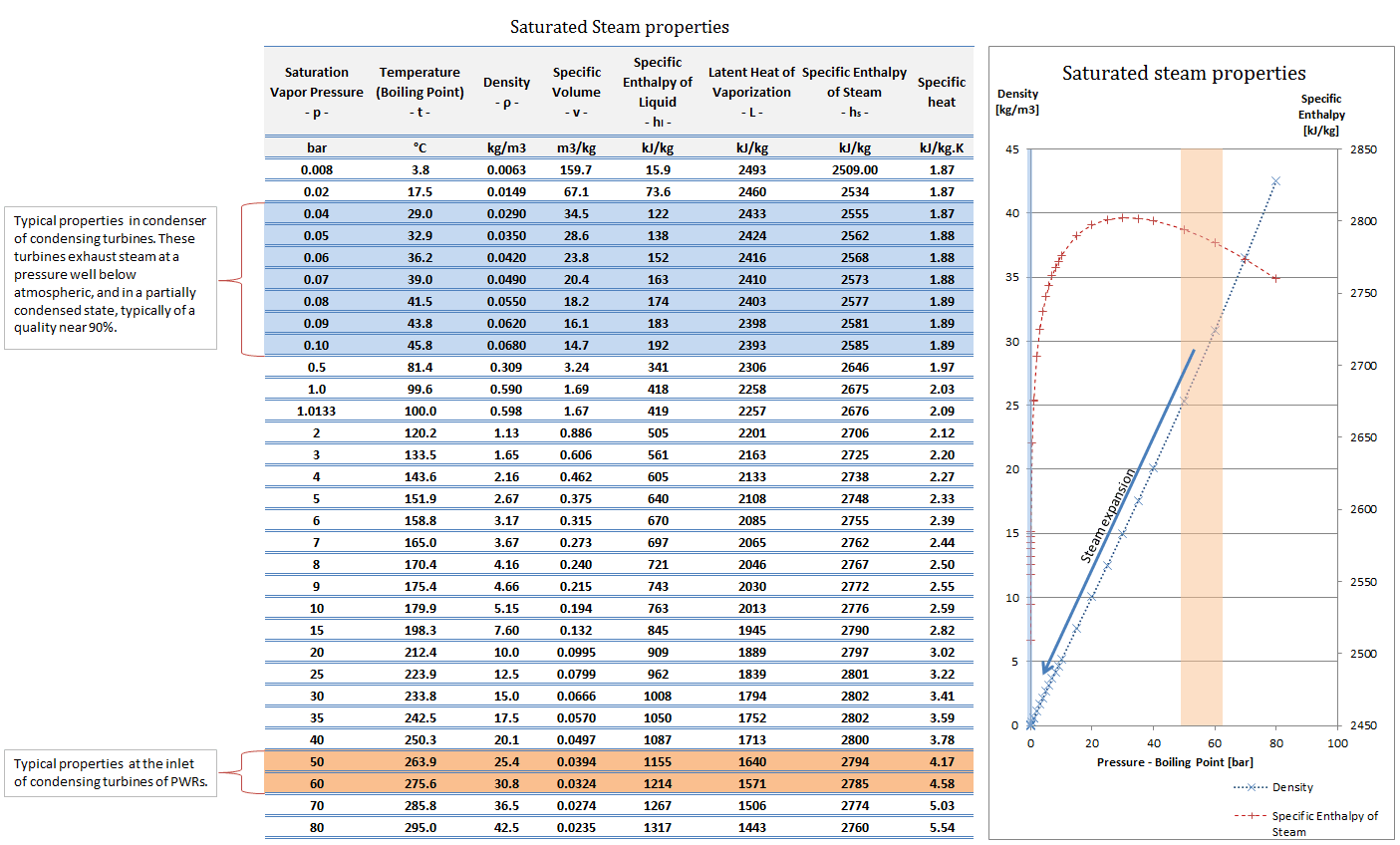
Steam Table Water Density Brokeasshome
http://nuclear-power.com/wp-content/uploads/2016/12/steam-properties-enthaply-density-volume-specific-heat-temperature.png
kg steam to m3 water - Steam Tables Online Calculator completely free Calculate propierties of wet saturated and superheated steam steam quality and more Thermodynamics calculator based on