is group 8 on the periodic table reactive The alkali metals found in group 1 of the periodic table formally known as group IA are so reactive that they are generally found in nature combined with other elements The alkali metals are shiny soft highly
A group is a vertical column down the periodic table while a period is a horizontal row across the table Both groups and periods reflect the organization of electrons in atoms Element atomic number increases as you The elements within the same group of the periodic table tend to exhibit similar physical and chemical properties Four major factors affect reactivity of metals nuclear charge atomic radius shielding effect and
is group 8 on the periodic table reactive

is group 8 on the periodic table reactive
https://i0.wp.com/www.periodictableprintable.com/wp-content/uploads/2022/07/where-are-metals-located-on-the-periodic-table-with-images-4.png

Halogen Facts Definition Properties Uses Britannica
https://cdn.britannica.com/45/7445-050-CA28EA33/version-periodic-table-elements.jpg
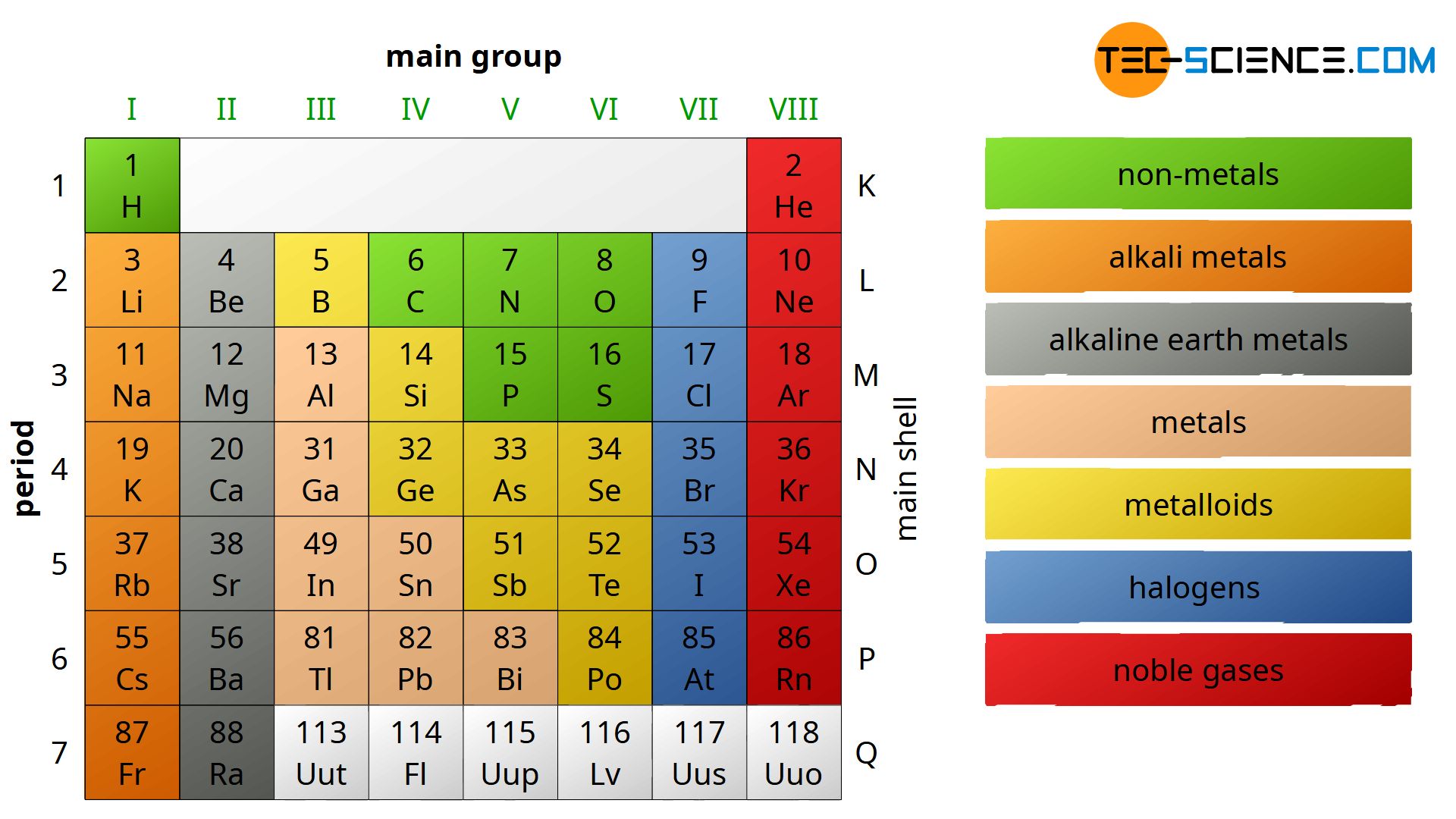
Periodic Table Of Chemical Elements Tec science
https://www.tec-science.com/wp-content/uploads/2021/02/en-periodic-table-main-group.jpg
The chemistry of group 8 is dominated by iron whose high abundance in Earth s crust is due to the extremely high stability of its nucleus Ruthenium and osmium on the other Periodic trends in properties such as atomic size and ionic size ionization energy electron affinity and electronegativity illustrate the strong connection between the chemical
This could be used to follow up some work on the periodic table where the trends in reactivity in groups 1 and 7 have been identified It can be used as a differentiated activity for the more able students within a group The least reactive elements are those who have a full outermost valence shell ie they have 8 electrons in the outer shell so elements such as helium neon radon or the
More picture related to is group 8 on the periodic table reactive

How To Read The Periodic Table Groups Periods ChemTalk 2023
https://i0.wp.com/chemistrytalk.org/wp-content/uploads/2023/03/1558px-Periodic_table.svg.png
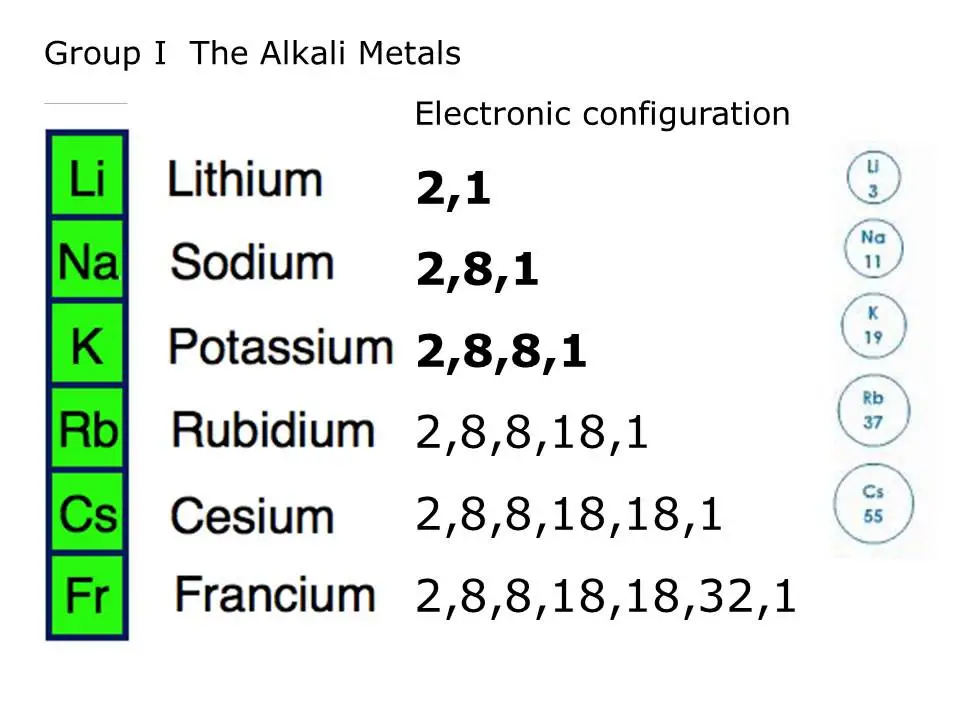
Elements Of S block Properties Of The First Group Elements 1A Alkali
https://www.online-sciences.com/wp-content/uploads/2018/02/Alkali-metals.webp
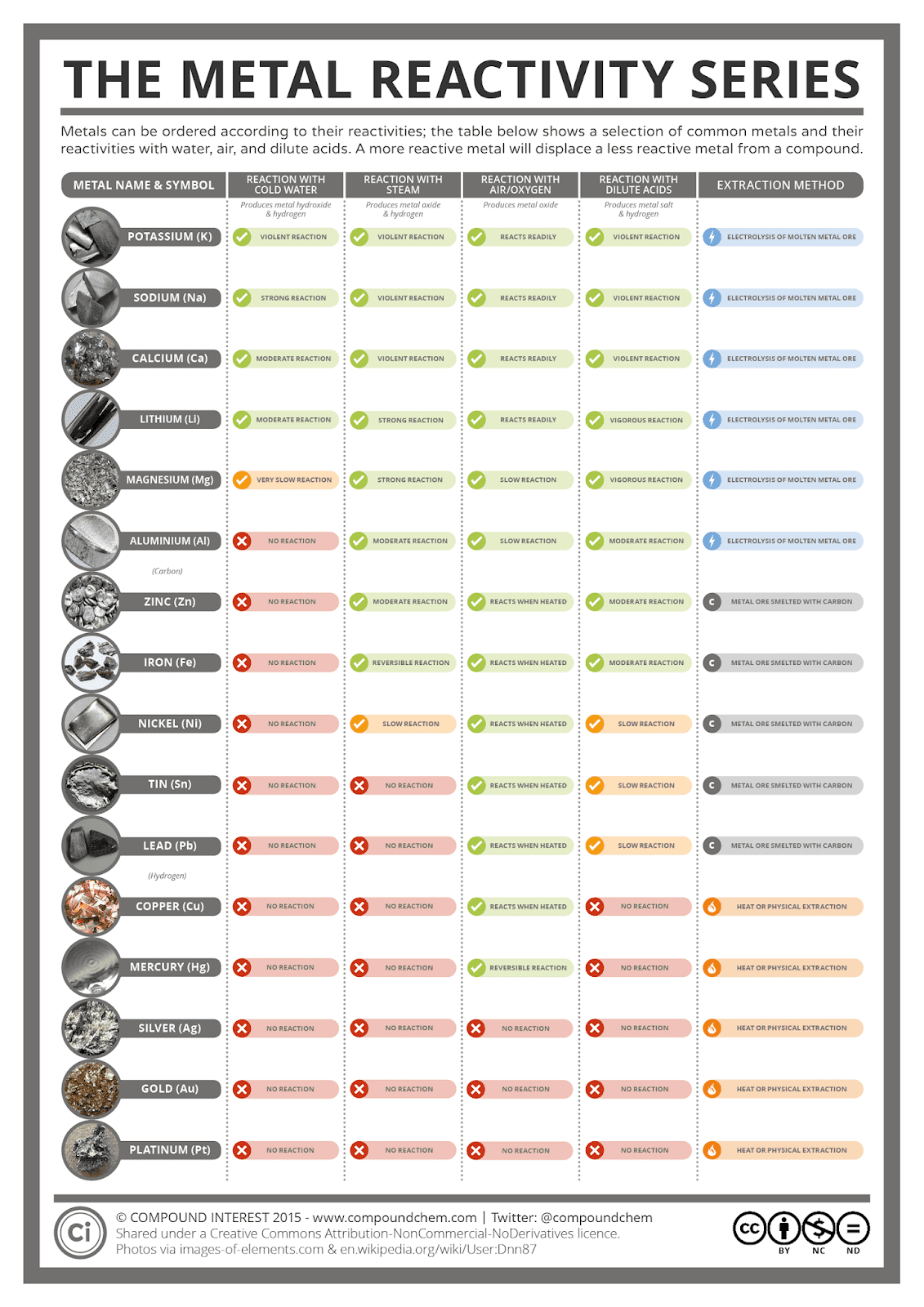
Reactivity Series Betsgerty
https://1.bp.blogspot.com/-nu4a7NdACq4/XjPTOAAeLpI/AAAAAAAAX6I/3I2DerYmpz075NW72eFmOPtLPZo_MDlNQCLcBGAsYHQ/s1600/The-Reactivity-Series-of-Metals.png
Reactivity series is a list of metals arranged in decreasing order of their reactivity Most reactive metals are at the top while the least reactive metals at the bottom For any two metals in the In this short series we look at what makes certain elements really reactive and others just not In this video Part 1 of 2 we take a look at metals wh
In chemistry the representative elements are the elements with atoms filling s and p electron orbitals Another name for the representative elements is the main group elements The group 7 elements are all reactive non metals They react with metals to form metal halides and with hydrogen to form acidic hydrogen halides Reactivity decreases down the group
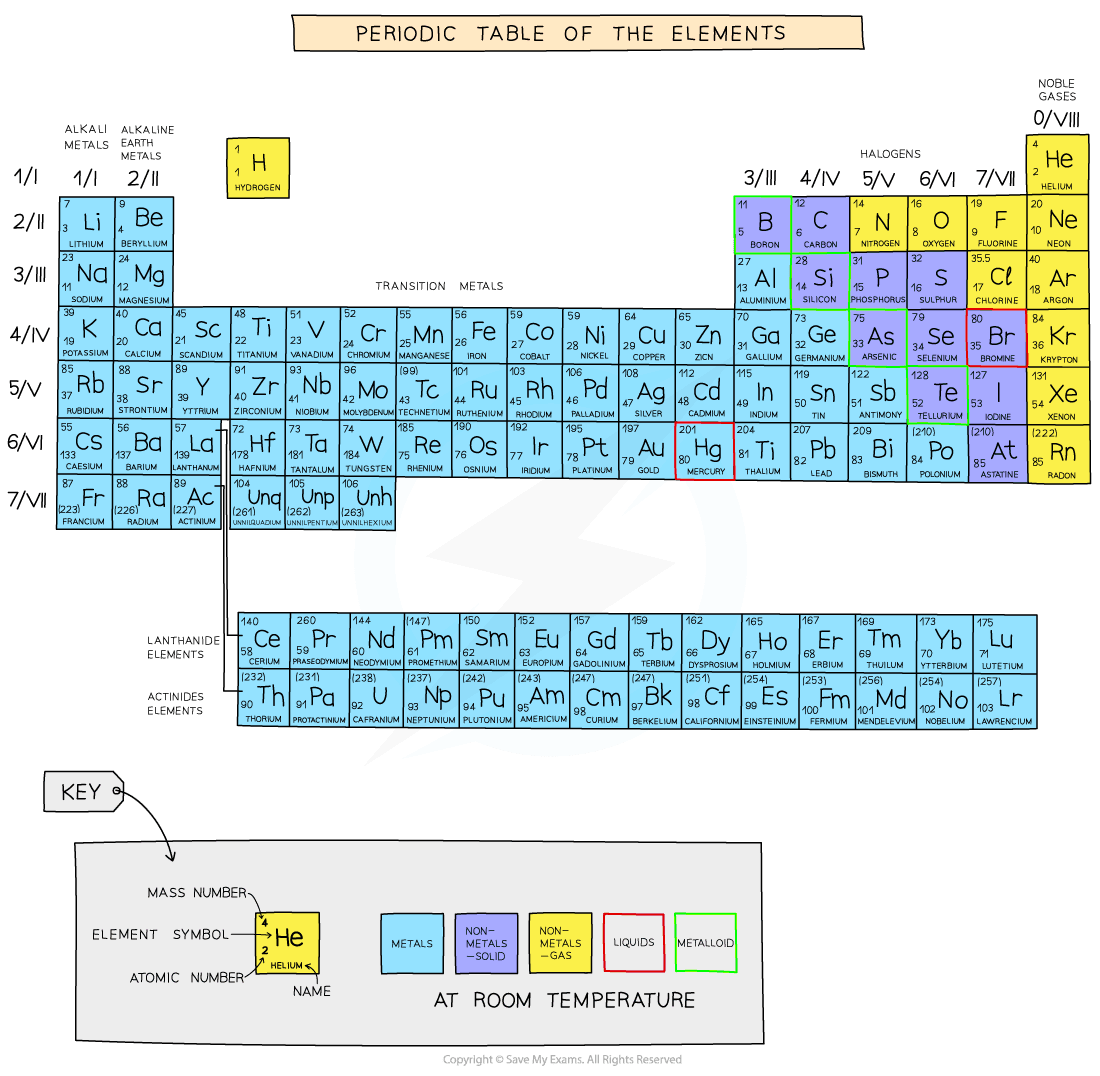
The Periodic Table Gidemy Class Notes
https://classnotes.gidemy.com/wp-content/uploads/sites/68/2021/12/Periodic-table.png?is-pending-load=1

Periodic Table And Periodicity The Elements
https://s2.studylib.net/store/data/015394108_1-063100f3c963a0f06248002fd8439afd-768x994.png
is group 8 on the periodic table reactive - The right most column on the periodic table Group 18 is labeled as Group 8A The elements which are boxed in purple in Figure PageIndex 1 are known as the noble