is 1 molar hcl dangerous Physical properties of hydrochloric acid such as boiling and melting points density and pH depend on the concentration or molarity of HCl in the aqueous solution They range from those of water at very low concentrations approaching 0 HCl to values for fuming hydrochloric acid at over 40 HCl Hydrochloric acid as the binary two component mixture of HCl and H2O has
Hazard class Acute toxicity oral Category 5 May be harmful if swallowed H303 Industrial exposure to hydrochloric acid vapors and mists is listed as a known human DANGER It causes severe skin burns and eye damage It is toxic if breathed in For a 15 minute exposure the concentration in the atmosphere should not exceed 8 mg m 3 Effects of
is 1 molar hcl dangerous

is 1 molar hcl dangerous
https://search-static.byjusweb.com/question-images/toppr_ext/questions/1311446_1347171_ans_bf8ed49d150e469fa5700c56caa41d98.jpg

How To Prepare 0 1M HCl Solution YouTube
https://i.ytimg.com/vi/tvA3TLhvmZY/maxresdefault.jpg
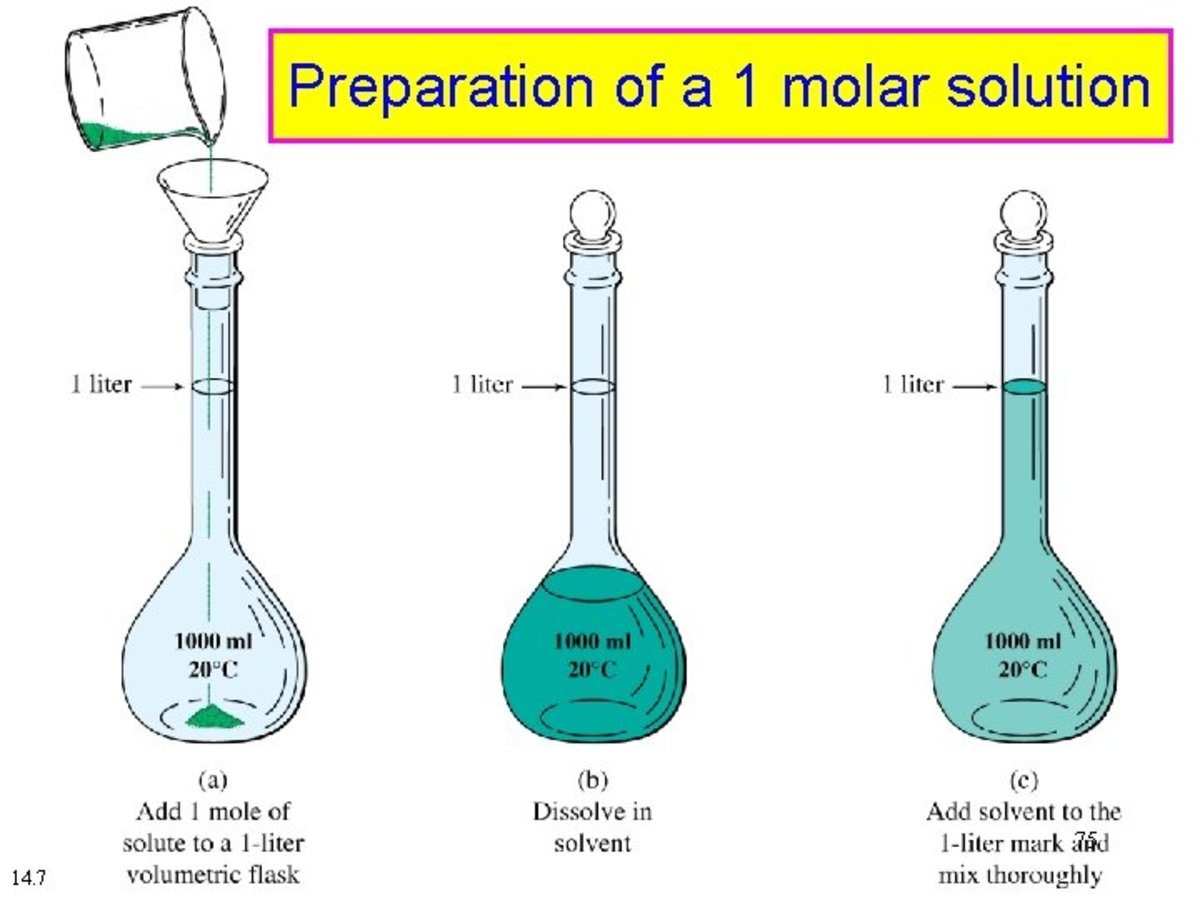
Molarity HubPages
https://images.saymedia-content.com/.image/t_share/MTg2OTcyMzI5NDU1MTk5NzM2/molarity.jpg
The compound hydrogen chloride has the chemical formula HCl and as such is a hydrogen halide At room temperature it is a colorless gas which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor Hydrochloric acid is a clear poisonous liquid It is a caustic chemical and highly corrosive which means it immediately causes severe damage to tissues such as burning on
Content source National Institute for Occupational Safety and Health Anhydrous hydrogen chloride Aqueous hydrogen chloride Hydrochloric acid Note Often used in an aqueous Hydrochloric acid is corrosive to the eyes skin and mucous membranes Acute short term inhalation exposure may cause eye nose and respiratory tract irritation and inflammation and
More picture related to is 1 molar hcl dangerous
/GettyImages-1091303816-601d1b45790b495d86be2a6522ea7b42.jpg)
Molars And Wisdom Teeth Function And Problems
https://www.verywellhealth.com/thmb/BgZn9aJVes-t_-ZEHwTCPbf5Oog=/1961x1529/filters:fill(87E3EF,1)/GettyImages-1091303816-601d1b45790b495d86be2a6522ea7b42.jpg

Conductometric Titration I Strong Acid HCl Versus Strong Base
https://i.ytimg.com/vi/K5qt0dWK-5c/maxresdefault.jpg

Calculate The Molecular Mass Of HCl The Molar Mass Of HCl
https://i.ytimg.com/vi/s3NpJ1NkkrY/maxresdefault.jpg
Why is hydrochloric acid so dangerous Because it s a strong acid Acids are termed weak or strong based on how readily they donate their hydrogen atoms Hydrochloric acid is formed when hydrogen chloride HCl hydrogen chloride is a really nasty dangerous gas but dissolved in water it makes a convenient acid hydrochloric acid for many applications in the lab or in industry When working with an HCl solutions in
HCl has a very low pH making it a strong acid A 1 Molar 1M solution of HCl in water has a pH of around 0 indicating its high acidity Stability While Hydrochloric Acid is Health Hazard Inhalation of fumes results in coughing and choking sensation and irritation of nose and lungs Liquid causes burns USCG 1999 Reactivity Profile HYDROCHLORIC

How To Prepare 0 01 N Hcl Solution In Pharma Pharmabeej
https://pharmabeej.com/wp-content/uploads/2023/04/0.01N-HCl-Preparation-1-1024x576.png

18 How Much Ml Of 0 1 Molar HCl Solution Is Required To Have A
https://toppr-doubts-media.s3.amazonaws.com/images/5468271/2c1d2320-5e1f-4967-b886-1e97b686d568.jpg
is 1 molar hcl dangerous - Hydrochloric acid is corrosive and concentrated forms often release acidic mists upon reaction with other substances that are also dangerous If the acid or mist come into