how to convert torr to atm The ideal gas law equation allows for the use of a wide variety of units as long as you correlate these units with those that express the gas constant R The ideal gas law equation looks like this PV nRT where P pressure the most commonly used units used to express pressure are atm mmHg torr Pa kPa bar V volume commonly used units are L
Approx 2 atmospheres The total pressure is the sum of the individual partial pressures of each gas The gases were quoted with unrealistically different units 1 atmosphere 760 mm Hg 760 Torr The torr is a unit of measure for barometric pressure and it is equal to 1mmHg Barometric pressure is the same as atmospheric pressure which is the is the force per unit area exerted on a surface by the weight of air above that surface in the atmosphere of Earth or another planet It can be measured in mmHg torr atmospheres atm Pascals Pa bars inHg
how to convert torr to atm
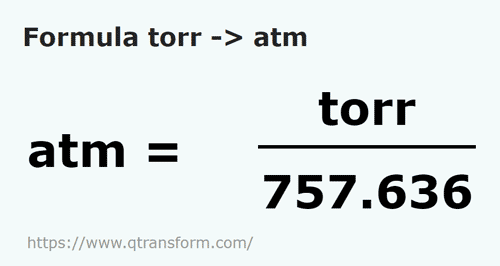
how to convert torr to atm
https://www.qtransform.com/imagini/formula/presiune/formula-torr-atm.gif
Mmhg To Atm Bloggerfasr
http://bloggerfasr541.weebly.com/uploads/1/2/5/3/125303143/200848586.jpg

Convert 0 921 Atm To Torr Pressure Conversion Practice YouTube
https://i.ytimg.com/vi/6qfRYL6S9A0/maxresdefault.jpg
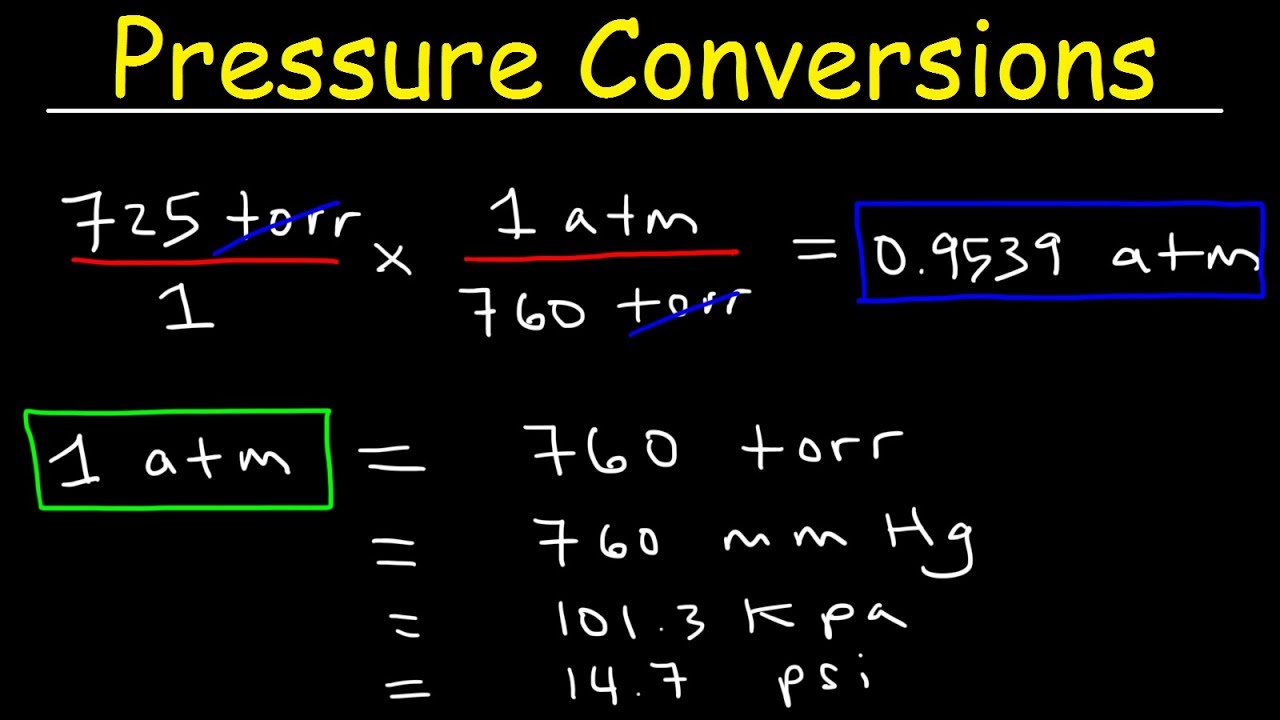
You probably should not do so Torr or mm Hg are a very convenient measurement to use ON EARTH 1 atmosphere of pressure will support a column of mercury 760 mm high This is a very visual and very convenient way to measure atmospheric pressure in a laboratory Of course on Venus which has a much thicker atmosphere the atmosphere 4712 torr Toconvert atm to torr first identify the equivalence statement of the two units involved that is 1 atm 760 torr since we already identify the desired unit to be torr the conversion factor would be 760 t0rr 1 atm therefore 6 2 cancel atm x 760 t0rr 1 cancel atm 4712 trr
P 1 75 atm kPa mmHg 1 atm 760 mm Hg We can write the two conversion factors 1 atm 760 mm Hg or 760 mm Hg 1 atm 1 75 atm x 760 mm Hg 1 atm 1330 mm Hg 1 atm 101325 pa We can write two conversion factors 1 atm 101325 pa or 101325 pa 1 atm 1 75 atm x 101325 pa 1 atm 177319 pa or 177 319 kPa 0 055 atm 42 torr We re asked to convert a given pressure value to two other units atm and torr
More picture related to how to convert torr to atm

Unit Conversion Torr To Atm YouTube
https://i.ytimg.com/vi/IVIJ5pN8J_s/maxresdefault.jpg

Solved Convert 836 Torr To Atmospheres 836 Torr Atm Chegg
https://media.cheggcdn.com/media/546/546f5299-a266-4cfb-8919-4ce16831b77c/phprUMjaW.png

Solved Convert 836 Torr To Atmospheres Atm 836 Torr Convert Chegg
https://media.cheggcdn.com/media/107/107c7647-0961-465b-b417-ab90bd1b4ad7/image.png
A small research submarine with a volume of 1 2x10 5 L has an internal pressure of 1 0 atm and an internal temperature of 150 C If the submarine descends to a depth where the pressure is 150 atm and the temperature is 30 C what will the volume of the gas inside be if the hull of the submarine breaks In this case we can look up the value of mm Hg for 1 atm Atmosphere unit 1 atm 760 mm Hg Then we can turn this into a conversion factor 1 atm 760 mm Hg or 760 mm Hg 1 atm We can now apply the conversion by starting with the unit we have and selecting the factor that cancels that unit and leaves the one we
[desc-10] [desc-11]

Pressure 1 Atmosphere 1 Bar 760 Mm Hg 760 Torr 100 000 Pa Ion
https://static.fdocument.org/img/1200x630/reader023/reader/2020111616/551b0dc1550346f70d8b5d59/r-1.jpg?t=1613210888

PPT Converting Pressures PowerPoint Presentation Free Download ID
https://image2.slideserve.com/4841665/converting-pressures-l.jpg
how to convert torr to atm - You probably should not do so Torr or mm Hg are a very convenient measurement to use ON EARTH 1 atmosphere of pressure will support a column of mercury 760 mm high This is a very visual and very convenient way to measure atmospheric pressure in a laboratory Of course on Venus which has a much thicker atmosphere the atmosphere