how much is 1 mol The mole is related to the mass of an element in the following way one mole of carbon 12 atoms has 6 02214076 10 23 atoms and a mass of 12 grams In comparison one mole of oxygen consists by definition of the same number of atoms as carbon 12 but it has a mass of 15 999 grams
Symbol mol Mole is the SI unit used to measure how many molecules or atoms there are One mole is around 600 sextillion molecules Scientists use this number because 1 gram of hydrogen is around 1 mole of atoms The exact value of one mole is 6 022 140 78 1023 One mole of a substance is equal to 6 022 10 units of that substance such as atoms molecules or ions The number 6 022 10 is known as Avogadro s number or Avogadro s constant The concept of the mole can be used to convert between mass and number of particles Created by Sal Khan
how much is 1 mol
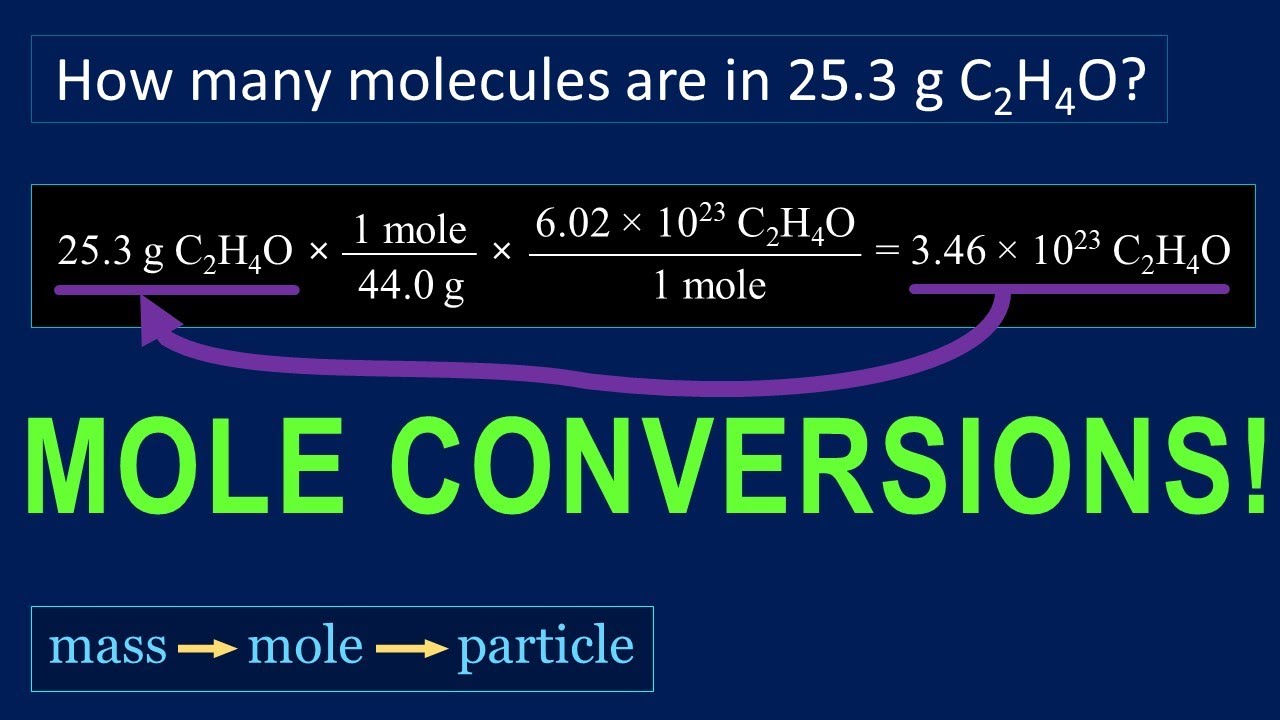
how much is 1 mol
https://i.ytimg.com/vi/t1pjGbwqt9o/maxresdefault.jpg

Moles To Particles Calculator Tewsgw
https://i.ytimg.com/vi/mpHQZ1PrkUI/maxresdefault.jpg

Moles To Grams Conversion Examples
https://sciencenotes.org/wp-content/uploads/2022/08/Moles-to-Grams-Conversion-1024x683.png
Key Takeaways Mole in Chemistry The mole is an SI unit used to measure the amount of any substance The abbreviation for mole is mol One mole is exactly 6 02214076 10 23 particles The particles could be something small like electrons or atoms or something large like elephants or stars What Is a Mole To calculate the number of moles of an element use the formula n m M where m is the Mass of the element and M is the Molecular weight How much is 1 mole to grams The mass of one mole of a substance is equal to its molar mass in grams
One mole of isotopically pure carbon 12 has a mass of 12 g For an element the molar mass is the mass of 1 mol of atoms of that element for a covalent molecular compound it is the mass of 1 mol of molecules of that compound for an ionic compound it is the mass of 1 mol of formula units One mole of any substance has a mass equal to its atomic or molecular weight in grams For example one mole of carbon 12 atoms weighs 12 grams while one mole of water H2O molecules weighs 18 grams 2 hydrogen atoms weigh 1 gram each and 1 oxygen atom weighs 16 grams This relationship between moles and mass is known as the
More picture related to how much is 1 mol

What Is The Energy Of Each Of The Following Photons In Kilojoules Per
https://i.ytimg.com/vi/jHc-yYJchrU/maxresdefault.jpg

As A 1 00 Mol Sample Of A Monatomic Ideal Gas Expands Adiabatically
https://d1hj4to4g9ba46.cloudfront.net/questions/1902277_1859930_ans_fd9a30118a78475c8542dcaeae3efceb.jpeg

The Mole UCalgary Chemistry Textbook
https://chem-textbook.ucalgary.ca/wp-content/uploads/2023/03/CNX_Chem_03_02_moles.jpg
A mole mol is a number of things equal to the number of atoms in exactly 12 g of carbon 12 Experimental measurements have determined that this number is very large 1 mol 6 02214179 1023 things Understand that a mole means a number of things just like a dozen means a certain number of things twelve in the case of a dozen Because 1 N 2 molecule contains 2 N atoms 1 mol of N 2 molecules 6 022 10 23 molecules has 2 mol of N atoms Using formulas to indicate how many atoms of each element we have in a substance we can relate the number of moles of molecules to the number of moles of atoms
[desc-10] [desc-11]

Waarom We Vanavond Allemaal Wie Is De Mol Kijken ja Jij Ook
https://www.psychologiemagazine.nl/wp-content/uploads/2018/03/mol-scaled.jpg
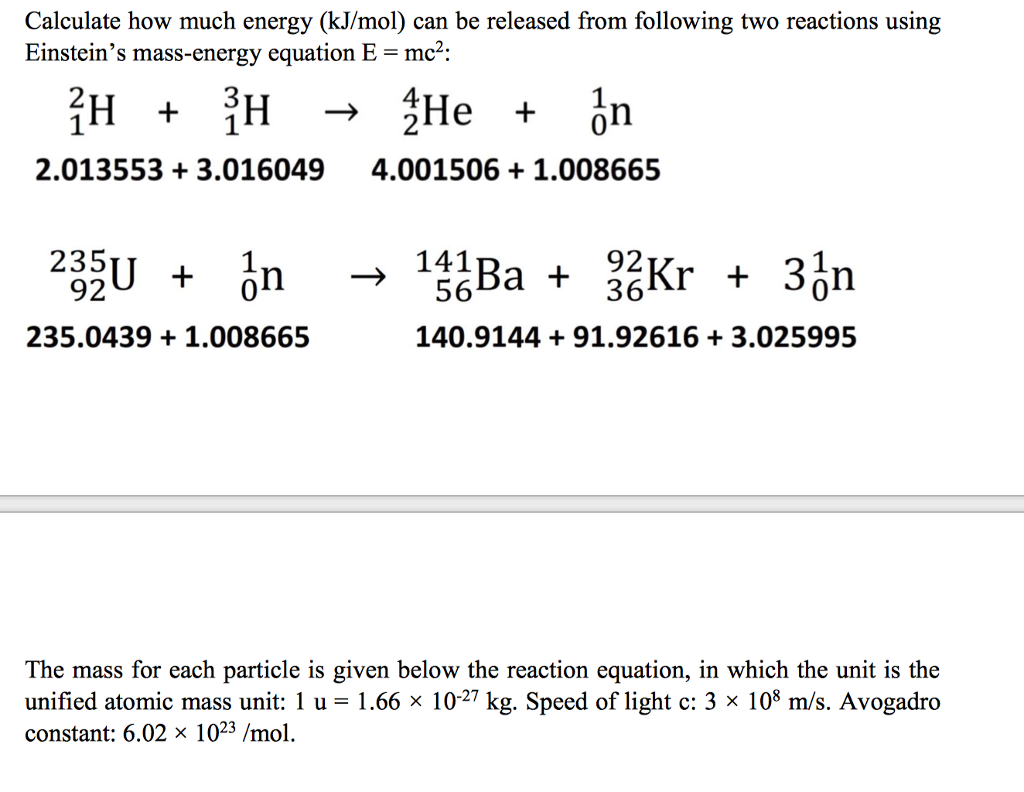
Solved Calculate How Much Energy kJ mol Can Be Released Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/674/674a34ee-1cf6-45bb-b4c7-b6a4373b4ffa/phpfKvgMJ.png
how much is 1 mol - One mole of any substance has a mass equal to its atomic or molecular weight in grams For example one mole of carbon 12 atoms weighs 12 grams while one mole of water H2O molecules weighs 18 grams 2 hydrogen atoms weigh 1 gram each and 1 oxygen atom weighs 16 grams This relationship between moles and mass is known as the