how many moles in 1 ml of water Apply the conversion formula Multiply the number of moles by the molar mass Divide by the product of density and concentration Check units to ensure consistency For converting 0 75
One mole of water is about 18 milliliters This is the volume of a few drops of water 3 65 teaspoons 1 2 tablespoons or 0 018 liters It s not a large volume yet it contains 6 022 x Water H 2 O is made from 2 atoms of hydrogen and 1 atom of oxygen A mole of water molecules would be 2 moles of hydrogen atoms plus
how many moles in 1 ml of water
/mole-big-578e5adf5f9b584d2015e449.jpg)
how many moles in 1 ml of water
https://www.thespruce.com/thmb/HRpuYSaKxclgAptW3cJb1_Juiwo=/2120x1415/filters:fill(auto,1)/mole-big-578e5adf5f9b584d2015e449.jpg

How To Get Rid Of Moles In Your Yard And Garden
https://www.reviewsroot.com/wp-content/uploads/2020/04/how-to-get-rid-of-moles-in-yard-scaled.jpg
:max_bytes(150000):strip_icc()/__opt__aboutcom__coeus__resources__content_migration__mnn__images__2018__11__mole-with-earthworm-01-12c0fbf7ccf24a28ae109ecd92406041.jpg)
How To Get Rid Of Moles In Your Yard
https://www.treehugger.com/thmb/RPYrCeQdyvrFpkiLs9pNNqAQUWA=/4000x2669/filters:no_upscale():max_bytes(150000):strip_icc()/__opt__aboutcom__coeus__resources__content_migration__mnn__images__2018__11__mole-with-earthworm-01-12c0fbf7ccf24a28ae109ecd92406041.jpg
MOLES FROM VOLUME OF PURE LIQUID OR SOLID There are two steps Multiply the volume by the density to get the mass Divide the mass by the molar mass to get Water has a molarity of 55 5 M 1 liter of water weighs 1000 g and as molarity is the number of moles per liter finding the molarity of water is the same as finding the number of moles of water in 1000 g We therefore
Convert amount of Water in moles to volume and weight using its molecular weight and density Materials moles to volume and weight calculator The unit of molarity M or mol L simply tells you how many moles of solute are dissolved in one liter of solution For example a 1 M 1 mol L solution contains 1 mole of solute in every liter of
More picture related to how many moles in 1 ml of water

9 Easy to Make Mole Repellents
https://www.tipsbulletin.com/wp-content/uploads/2019/02/natural-mole-repellent-t1.jpg

Mole History And Some Interesting Facts
https://animalia-life.com/data_images/mole/mole5.jpg

Moles Are Perfectly Suited For Their Underground Lifestyle Forest
https://www.reconnectwithnature.org/getmedia/bef9a00c-4039-4232-90f7-c8f9ebe93581/Eastern-mole-Shutterstock.jpg?width=1500&height=1000&ext=.jpg
A mole in chemistry is just a group word for an amount of mass you want to use in a chemical reaction Essentially a mole is a standard unit of measurement that represents 6 022 1 18 moles of water contains 1 6 022 1023 18 3 34 1022molecules
Thus we can read this reaction as two moles of hydrogen react with one mole of oxygen to produce two moles of water By the same token the ratios we constructed to describe a Calculate the quantity of substance in moles using mass and chemical formula Plus learn six different formulas to calculate moles
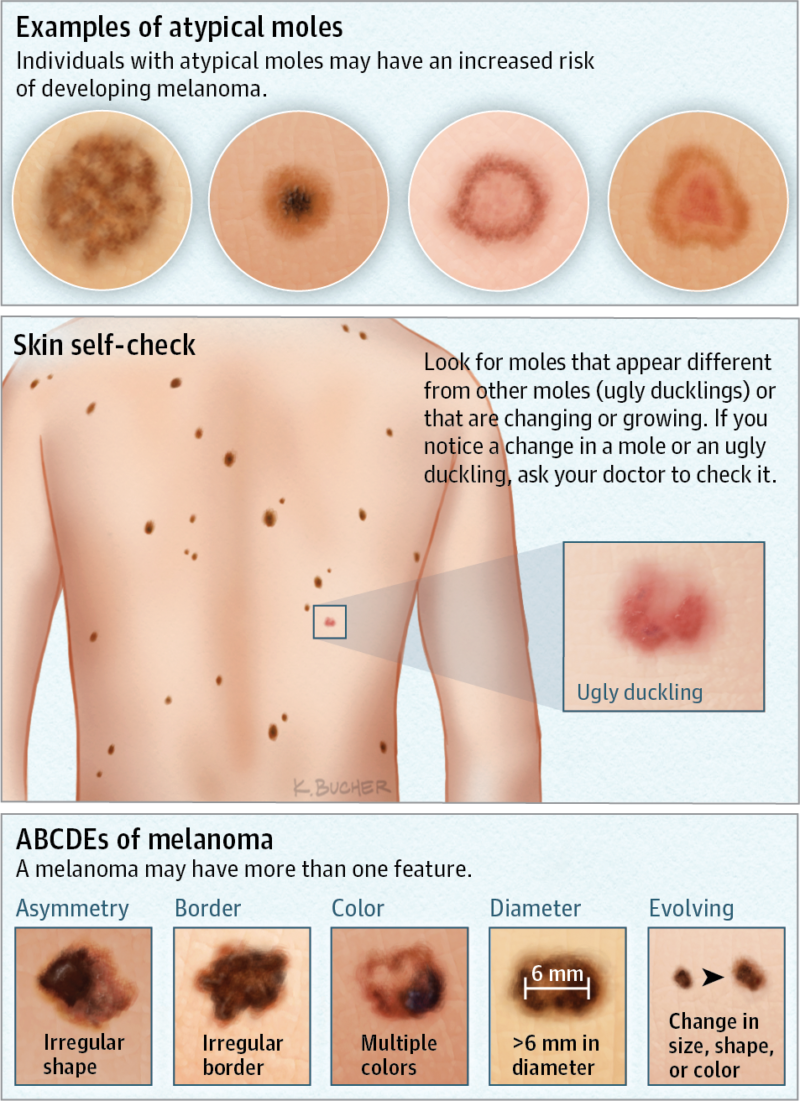
Mole Evaluation Skin Care Center
https://skincarectr.com/wp-content/uploads/2019/04/atypical-moles-800x1101.png
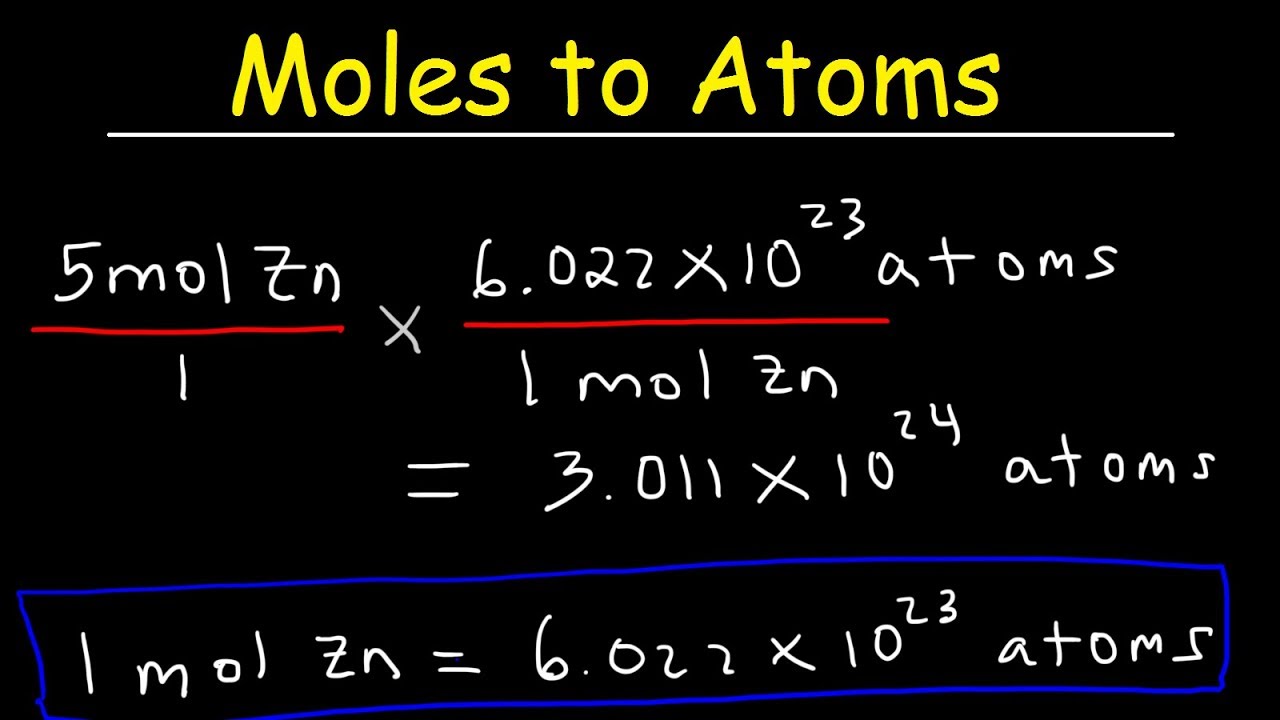
Moles To Particles Calculator Tewsgw
https://i.ytimg.com/vi/mpHQZ1PrkUI/maxresdefault.jpg
how many moles in 1 ml of water - The unit of molarity M or mol L simply tells you how many moles of solute are dissolved in one liter of solution For example a 1 M 1 mol L solution contains 1 mole of solute in every liter of