how many grams of solute dissolves in 100 ml of water When a solution is saturated and excess solute is present the rate of dissolution is exactly equal to the rate of crystallization Figure PageIndex 1b Using the value just stated a saturated aqueous
Used to determine the mass of solute in 100g 100 ml of water at a given temperature Definitions On the line saturated full can not hold anymore solute Below the line unsaturated can hold more solute Above the This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating grams per ml to moles You can also
how many grams of solute dissolves in 100 ml of water
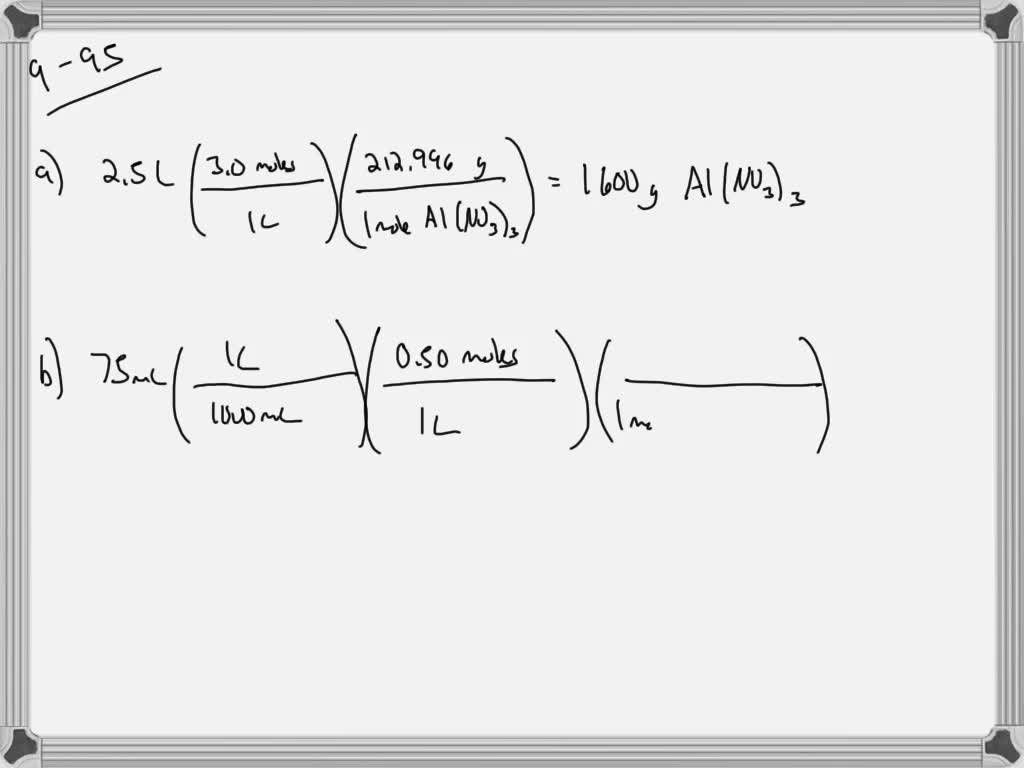
how many grams of solute dissolves in 100 ml of water
https://cdn.numerade.com/previews/a4f02a4a-bf7e-429d-aaa4-dbe7836bd3af_large.jpg

Calculate The Molarity Of NaOH In The Solution Prepared By Dissolving
https://i.ytimg.com/vi/LKZgxdGxS30/maxresdefault.jpg

Solutions Molarity From Grams Of Solute YouTube
https://i.ytimg.com/vi/zTZGD6SGdPk/maxresdefault.jpg
In most instances a 5 by volume solution of a solid will mean 5 g of the solute dissolved in 100 ml of the solvent Example PageIndex 3 Fish like all This online calculator can calculate the molar concentration of a solute in a solution or mass of a solute in a solution with a specific molar concentration
How many grams of solute should be added in 100g water to get a solution of density 1 2g ml and strength 5 w v 5g 6g 4 35g 4 17g A 5g B 6g C 4 17g D 4 35g At the new temperature the solubility limit in 100 mL of water is 244 g glucose With only 100 g of glucose dissolved the solution is now unsaturated If we next cool the mixture
More picture related to how many grams of solute dissolves in 100 ml of water
Solved Calculate The Mass In Grams Of Solute Needed To Prepare 100 ML
https://www.coursehero.com/qa/attachment/15777403/
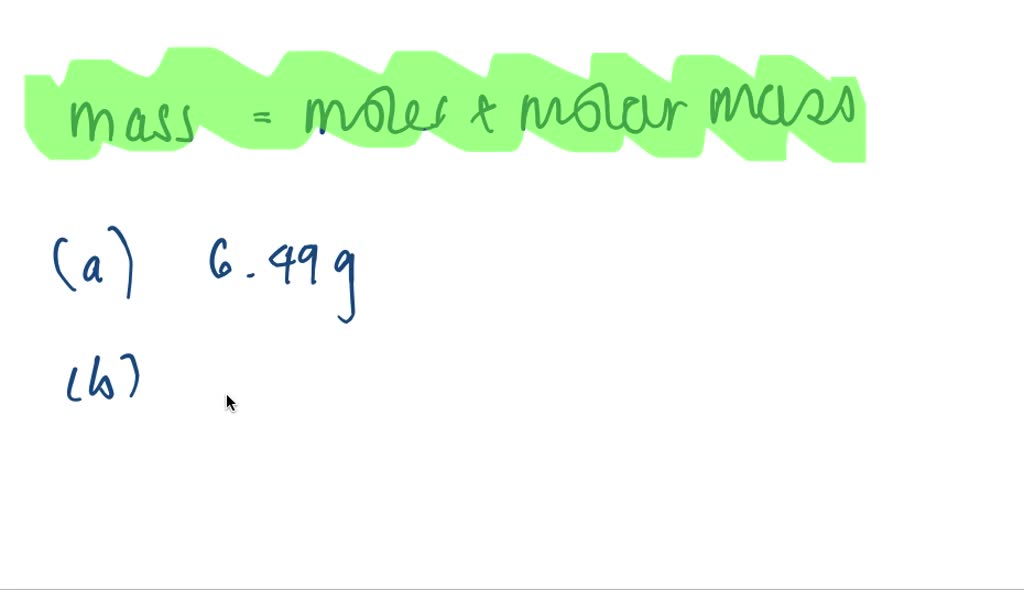
SOLVED Determine How Many Grams Of Each Of The Following Solutes Would
https://cdn.numerade.com/previews/504eb686-5904-438a-994e-182f08fd77a8_large.jpg
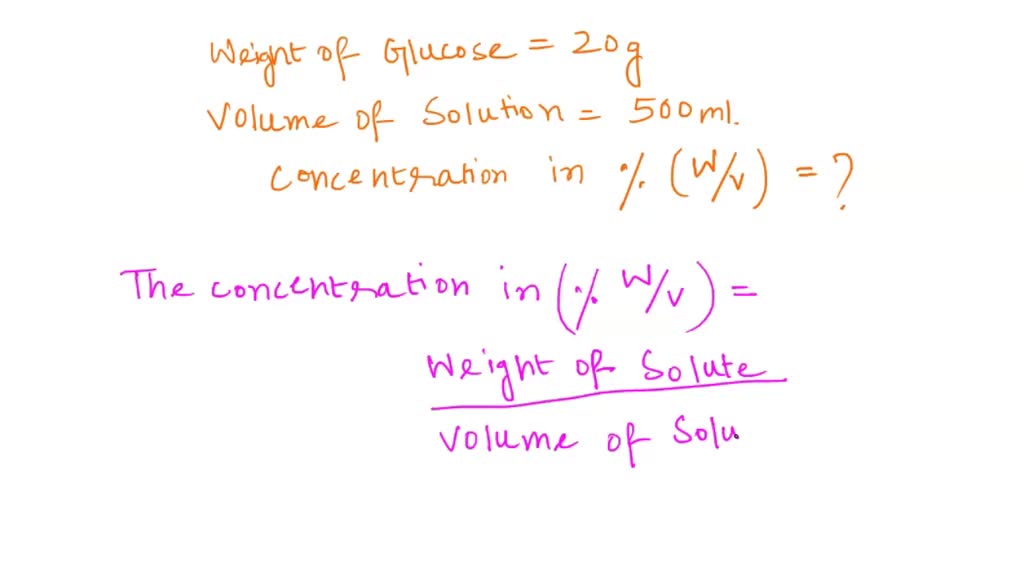
SOLVED Given The Amount Of Solute And Solvent Calculate The
https://cdn.numerade.com/ask_previews/23684fec-243c-4a00-ba0c-8179d6cd18bb_large.jpg
Solubility is often measured in grams of solute per 100 text g of solvent The solubility of sodium chloride in water is 36 0 text g per 100 text g of water at 20 text o text C A solution was prepared by dissolving 100 mg of protein X in 100 ml of water Molecular weight of protein X is 15 000 Da Avogadro s number 6 022x 1023 Calculate the
Calculate the solubility of sodium nitrate if 22 g of the salt is dissolved in 25 g of water Since we know the amount of sodium nitrate that dissolves in 25 grams of water we can easily calculate its solubility in 100g of water A solute is usually considered to be slightly soluble or sparingly soluble in water if between 0 1 and 1 0 g can be dissolved in 100 mL of water The solubility table below

Solubility Chemistry Diagram
https://cdn1.vectorstock.com/i/1000x1000/24/65/dissolving-solids-solubility-chemistry-vector-16082465.jpg
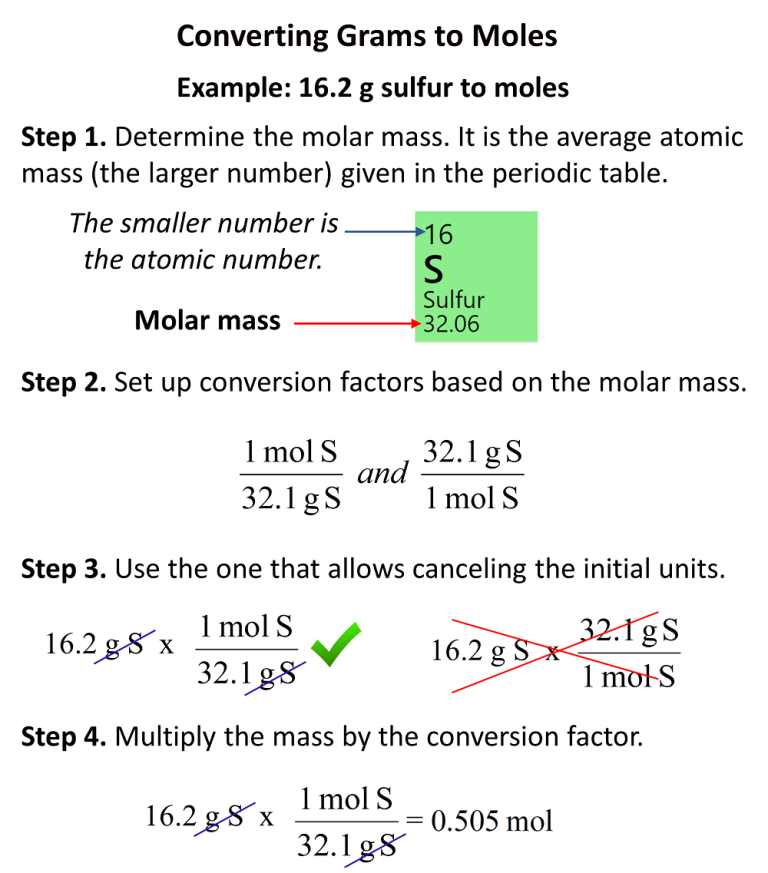
How To Convert Grams To Moles Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/12/Converting-grams-to-moles-easy-sumarry-steps-768x873.png
how many grams of solute dissolves in 100 ml of water - At the new temperature the solubility limit in 100 mL of water is 244 g glucose With only 100 g of glucose dissolved the solution is now unsaturated If we next cool the mixture