how many grams of nano3 can be dissolved in 300 ml of water at 10 c 74 According to Reference Table G how many grams of KClO 3 must be dissolved in 100 grams of H 2 O at 10 C to produce a saturated solution
This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating grams per ml to moles You can also calculate the NaNO3 because the higher line indicates that more NaNO3 can be dissolved 3 The line marks the saturation point of a solute in 100 grams of water If you have less grams dissolved than
how many grams of nano3 can be dissolved in 300 ml of water at 10 c

how many grams of nano3 can be dissolved in 300 ml of water at 10 c
https://i.ytimg.com/vi/ilFMAzMGWlA/maxresdefault.jpg
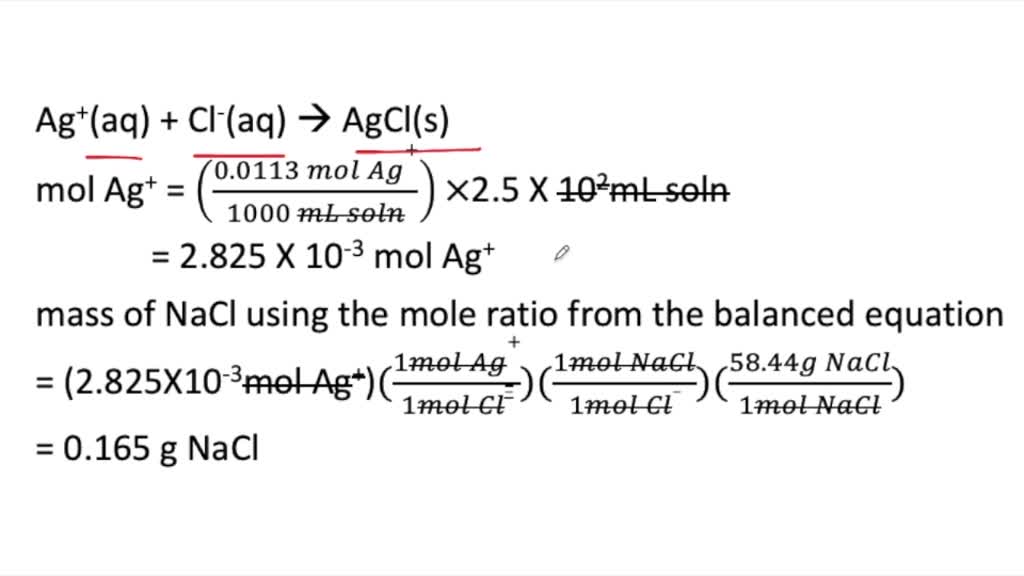
SOLVED For The Following Questions Use The Reaction Equation Below Pb
https://cdn.numerade.com/previews/ba08e6a6-6596-4c7d-b338-33b4c1d3c30b_large.jpg

Calculate The Molarity Of NaOH In The Solution Prepared By Dissolving
https://i.ytimg.com/vi/LKZgxdGxS30/maxresdefault.jpg
This online calculator can calculate the molar concentration of a solute in a solution or mass of a solute in a solution with a specific molar concentration If you wish to perform dilution factor or fold dilution calculations for solutions with mass per volume or weight per volume concentration units e g mg mL g L g L etc
Water temperature can have a significant effect on the solubility of compounds Refer to the chart below to find reference values per gram of common compounds and salts with chemical A 1 M solution is one in which exactly 1 mole of solute is dissolved in a total solution volume of exactly 1 L Using SI prefixes the concentration may also be expressed in different
More picture related to how many grams of nano3 can be dissolved in 300 ml of water at 10 c
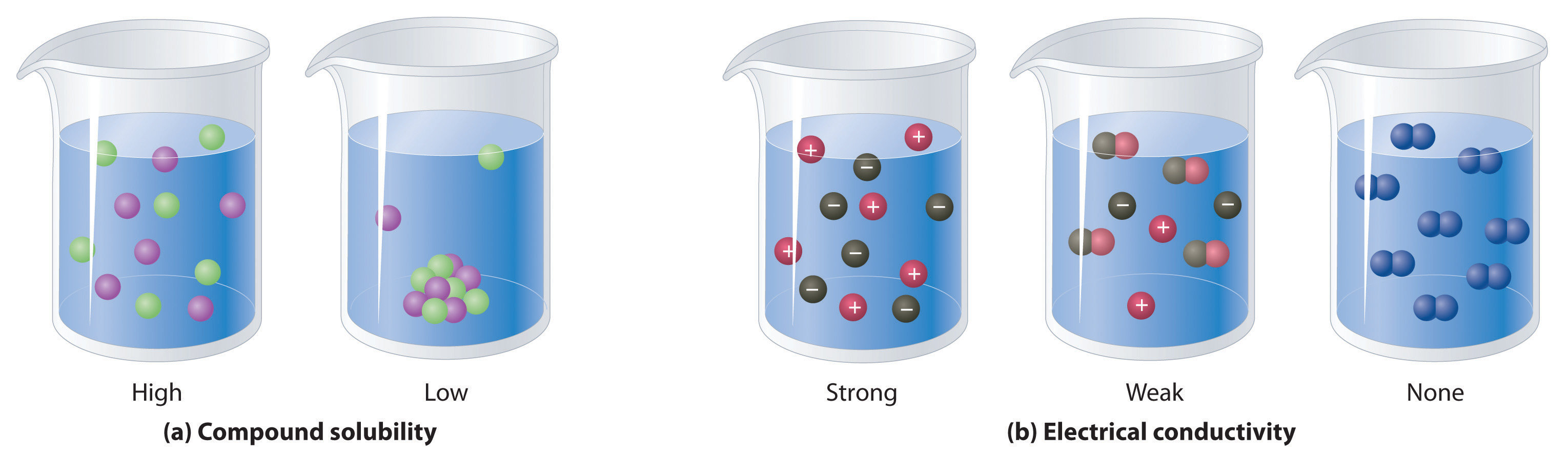
Aqueous Solutions
http://webmis.highland.cc.il.us/~jsullivan/principles-of-general-chemistry-v1.0/section_08/7e54d0c43e9597309aa9ecfdafbc69bb.jpg

SOLVED Calculate The Freezing Point And Melting Point Of A Solution
https://cdn.numerade.com/previews/c4a2c131-2847-4a6f-82ed-70c404c32cd1_large.jpg
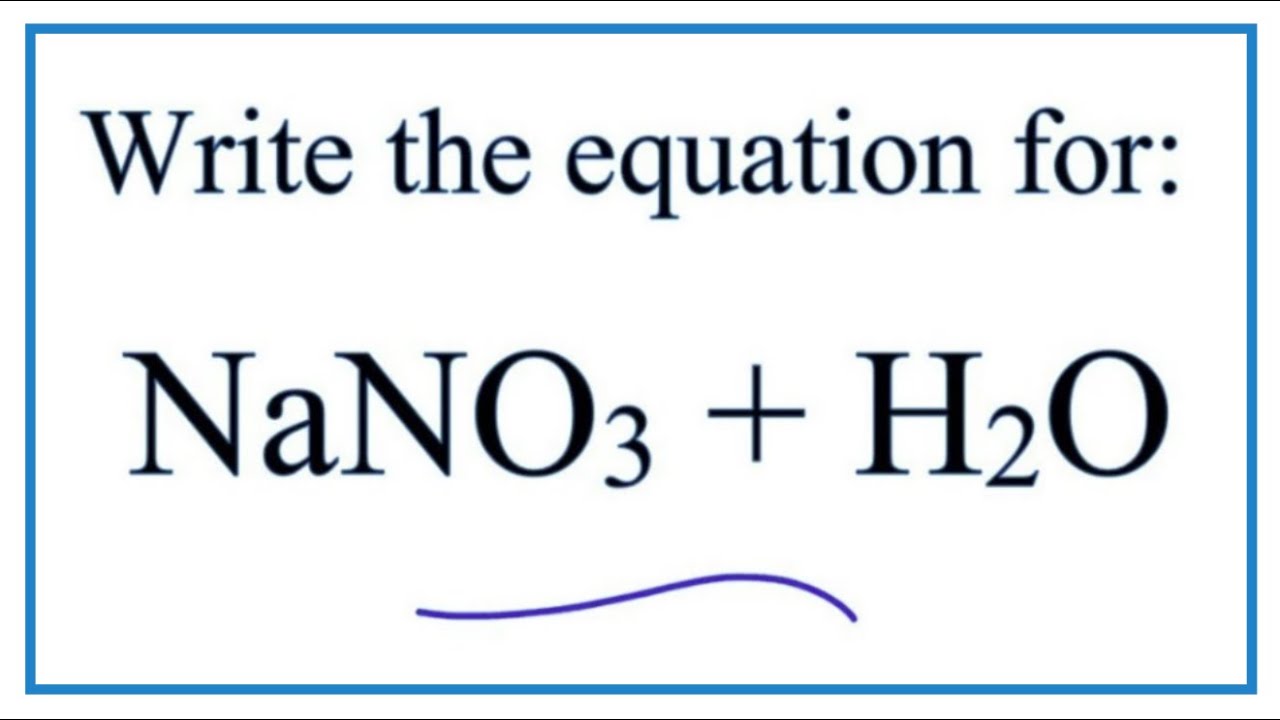
Equation For NaNO3 H2O Sodium Nitrate Water YouTube
https://i.ytimg.com/vi/o4DJGG5fNTQ/maxresdefault.jpg
If you have 50 g of NaNO3 in 100 g of water at 10 C you have an unsaturated solution How many more grams will you need to add for the solution to be saturated Your value of g 100g water Calculate the maximum number of grams of each solute that can be dissolved a potassium nitrate in 300 cm3 b sodium chloride in 1250 um3 c sodium nitrate in 50 crag of water at 80
The unit of molarity M or mol L simply tells you how many moles of solute are dissolved in one liter of solution For example a 1 M 1 mol L solution contains 1 mole of solute in every liter of This molarity calculator estimates the molar concentration of a solution by using the mass volume and molecular weight You can read more on the molar concentration and how to calculate the
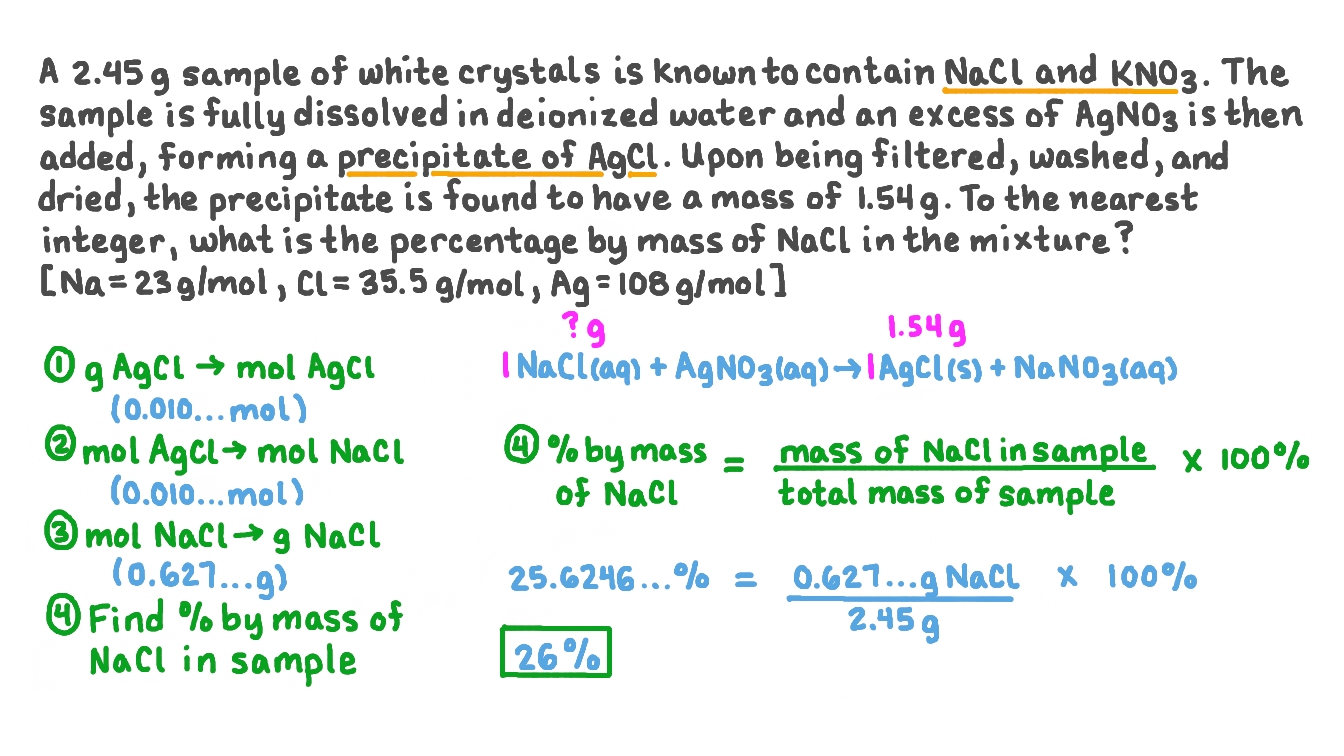
Question Video Using Precipitation Gravimetry To Calculate The
https://media.nagwa.com/608103791308/en/thumbnail_l.jpeg

How Many Grams Of NaNO3 Can Be Dissolved In 250 Grams Of Water At 10oC
https://us-static.z-dn.net/files/d89/d7b8d13a4307a9895e91622c1a7ba0a8.jpg
how many grams of nano3 can be dissolved in 300 ml of water at 10 c - C 0 208 M for the 200 mL solution and c 0 151 M for the 275 mL solution not rounded for significant figures Explanation molarity moles liter Since we have grams of