how many grams in 1 mole of co2 Convert grams to moles and moles to grams using a simply calculator Plus learn the g to mol formula and how to account for molar mass
Do a quick conversion 1 grams CO2 0 022722366761722 mole using the molecular weight calculator and the molar mass of CO2 In this video well learn to convert moles of CO2 to grams using both a simple formula and the factor label method For our practice problem we ll convert 25
how many grams in 1 mole of co2

how many grams in 1 mole of co2
https://i.ytimg.com/vi/mpHQZ1PrkUI/maxresdefault.jpg

1 Mole Of Water In Grams Cloudshareinfo
https://useruploads.socratic.org/PJGJQ5CoSyJlIYJ1htS6_image.jpg

How To Convert Molecules To Grams Mole Conversions YouTube
https://i.ytimg.com/vi/S7PjcuT13NU/maxresdefault.jpg
Use this grams to moles calculator whenever you want to know the number of moles of a substance with a specific mass Need to convert between moles molecular weight and mass You can do it here with our mole calculator
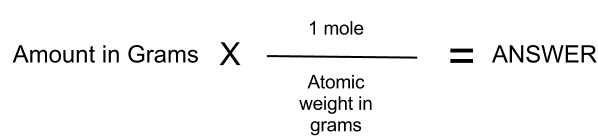
The number of moles of CO 2 is given by 1 Number of moles Given Mass Atomic Mass 1 Mass 44 Mass 44 g The mass of 1 mole of the substance is the molar mass that is This free moles to grams calculator can instantly convert moles to grams and determine how many atoms molecules are present in these grams
More picture related to how many grams in 1 mole of co2
How Many Grams In A Cup Cups To Grams The Big Man s World
https://thebigmansworld.com/wp-content/uploads/2022/04/cups-to-grams.jpg

As My Father Always Said when In Doubt Convert To Moles How To
https://i.pinimg.com/originals/0c/90/57/0c9057c93f809822aba1c5d724f6742b.jpg
Avogadro s Number The Key To Converting Grams To Atoms
https://h-o-m-e.org/wp-content/uploads/2023/04/grams-to-atoms-1.jpg
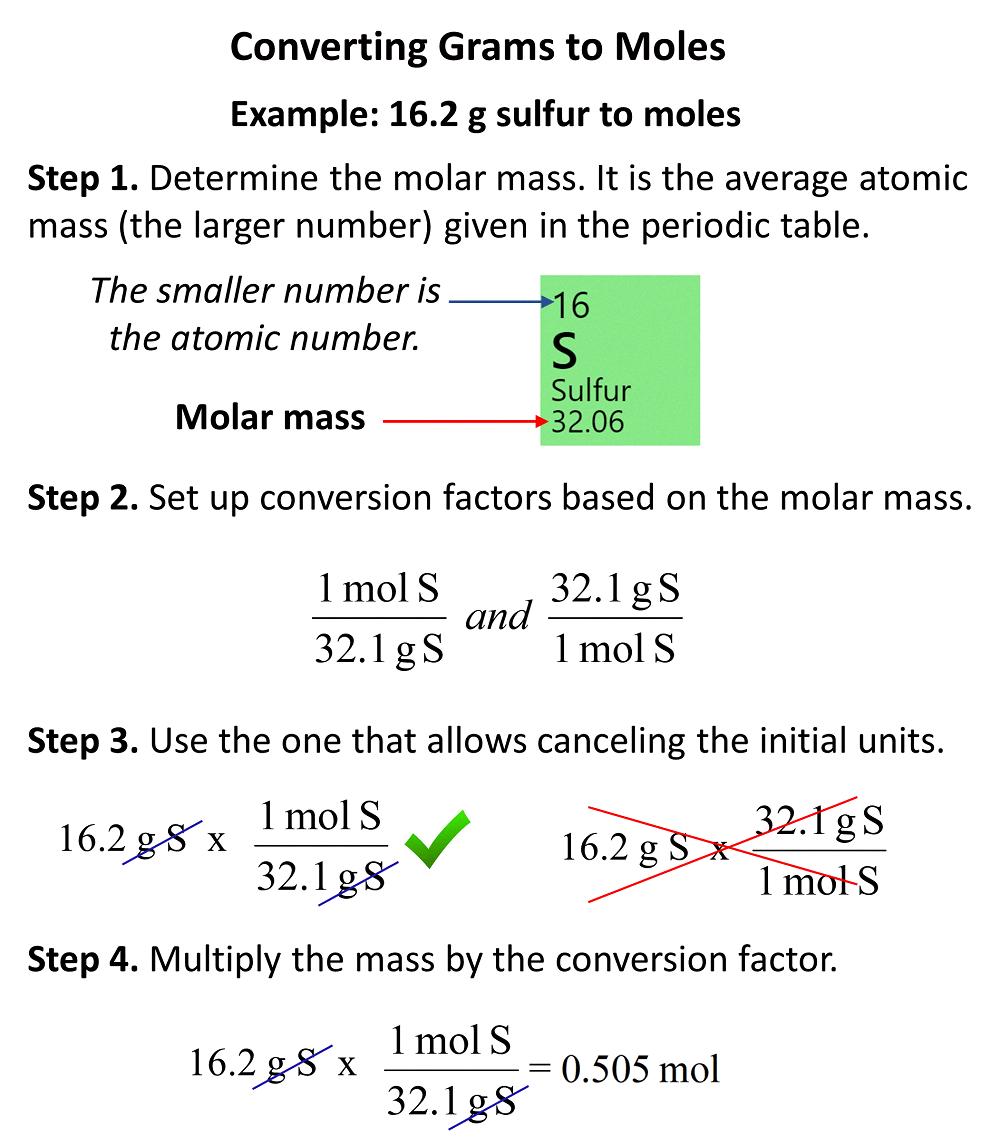
This grams to moles moles to grams calculator converts between moles and grams using a formula of a substance To calculate the grams of CO2 in 14 mol of the compound first find the molar mass of CO2 1 carbon atom 12 01 g mol 2 oxygen atoms 16 00 g mol each 44 01 g mol
In chemical stoichiometry the mole is defined as the mass of substance containing 1 Avogadro s Number N o of particles 1 N o 6 023xx10 23 units The molar mass of Carbon C is approximately 12 01 g mol and the molar mass of Oxygen O is approximately 16 00 g mol Since CO 2 has one Carbon atom and two

How Many Moles Of Oxygen Atoms Are Present In One Mole Of The Following
https://hi-static.z-dn.net/files/d2a/a712459994420a0fb674a2925e2d9688.jpg
Moles Grams Conversion Chemistry Fun Facts
http://chemistryfunfacts.weebly.com/uploads/5/2/2/4/5224712/4530648_orig.png?538
how many grams in 1 mole of co2 - Explanation The weight of CO2 is 44 grams per mole 1 x 12 grams mole for the carbon and 2 x 16 grams mole for the oxygen atoms