how is e cell calculated E cell E cell frac RT zF ln Q in which E cell is the potential for the reduction half reaction R is the universal gas constant 8 31 J x K 1 mol 1 T is temperature in Kelvins z is the number of electrons transferred in the reaction and Q is the reaction quotient of the overall reaction
When both reactants and products are in their standard states the relationship between G and E cell is as follows Delta G nFE cell label 20 5 5 A spontaneous redox reaction is characterized by a negative value of G which corresponds to a positive value of E cell The two are closely related in the sense that the standard cell potential is used to calculate for the cell potential in many cases Ecell Eo cell RT nFlnQ Other simplified forms of the equation that we typically see Ecell Eo cell 0 0257 n lnQ
how is e cell calculated
how is e cell calculated
https://media.cheggcdn.com/study/653/653e1324-362b-4ebe-9eb0-9f0225922047/image

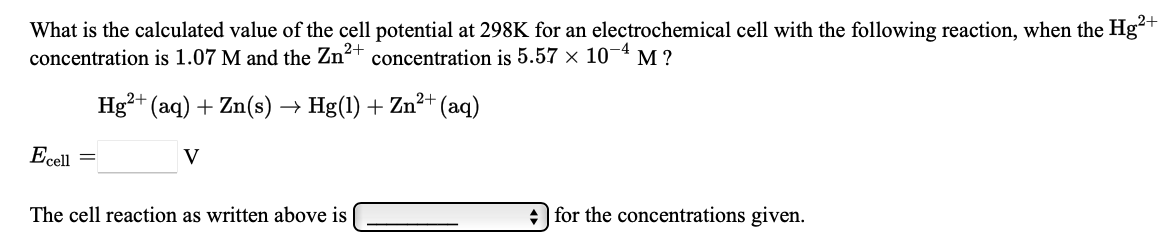
What Is The Calculated Value Of The Cell Potential At 298K For An
https://img.homeworklib.com/questions/891ebc40-62ff-11eb-9e51-41efe93a81b1.png?x-oss-process=image/resize,w_560

Dragon Ball Z What Would Happen If Cell Fought Majin Buu After Earth
http://www.youauthorus.com/wp-content/uploads/2016/01/cell-reddit-picture-test-copy.jpg
Determine E cell the cell potential at the non standard state conditions using the Nernst equation E cell E ocell RT nF ln Q E cell cell potential at non standard state conditions E ocell standard state cell potential R constant 8 31 J mole K T absolute temperature Kelvin scale F Faraday s constant 96 485 C mole e Worked example Calculating Ecell for the given cell Google Classroom About This video talks about the use of Nernst equation to calculate the cell potential for a given cell at a particular temperature other than the standard conditions Practice this concept khanacademy
You might need Calculator The following reactions occur in a cell At anode Cr s Cr A 3 0 005 M 3 e A At cathode H A 0 1 M e A 1 2 H A 2 1 bar Calculate E A cell Given E A o cell 0 74 V log The equation that you give E Cell More Positive E Potential Less Positive E potential only works if the less positive E potential is the reducing agent and the more positive E potential is the oxidizing agent when the reducing agent is negative and you haven t switched the sign this E Cell More Positive E Potential Less
More picture related to how is e cell calculated

Question Video Calculating The Standard Cell Potential For A Copper
https://media.nagwa.com/376151026358/en/thumbnail_l.jpeg

Electrochemical Cells And Thermodynamics Key calculated Shorthand
https://img.homeworklib.com/images/76b8bf74-2bea-4e40-a0f3-82097b3f11a8.png?x-oss-process=image/resize,w_560
Solved What Is The Calculated Value Of The Cell Potential At Chegg
https://media.cheggcdn.com/media/99b/99bde7ba-7899-43bb-95a9-69975a72c662/phpeIcvrH
E cell E cathode E anode E cell E cathode E anode where E cathode and E anode are the potentials of two different half cells functioning as specified in the subscripts As for other thermodynamic quantities the standard cell potential E cell is a cell potential measured when both half cells are under standard state conditions Using standard electrode potential data to calculate E cell Consider an electrochemical cell constructed from Ag aq Ag s and Mg 2 aq Mg s half cells For each half cell we can look up the value of the standard electrode potential E in a data book Ag aq e Ag s E 0 80V Mg 2 aq 2e Mg s E
Step 1 Understand the Nernst Equation The Nernst equation is a fundamental equation that relates the difference in potential between two half cells to their concentrations and temperature The equation is as follows E cell E0 cell RT nF lnQ Where E cell The cell potential calculated under non standard conditions Want to ace chemistry Access the best chemistry resource at conquerchemistry masterclass Need help with chemistry Download 12 Secrets t

Solved What Is The Calculated Value Of The Cell Potential At Chegg
https://media.cheggcdn.com/media/fa5/fa5e7ea6-0f2c-43c9-8a7d-f1e7d13ce8d5/phpPRFxqd.png

Cell Potential Formula HalleldMoses
https://media.nagwa.com/836152462980/en/thumbnail_l.jpeg
how is e cell calculated - You might need Calculator The following reactions occur in a cell At anode Cr s Cr A 3 0 005 M 3 e A At cathode H A 0 1 M e A 1 2 H A 2 1 bar Calculate E A cell Given E A o cell 0 74 V log