hess law of constant heat summation explain Hess s law rule proposed by Germain Henri Hess stating that the heat absorbed or evolved or the change in enthalpy in any chemical reaction is a fixed quantity and is
State and explain Hess s law of constant heat summation Enthalpy is an extensive property In general if enthalpy of an overall reaction A B along one route is r H and r H 1 r H 2 r H 3 represent enthalpies of Hess s Law of Constant Heat Summation or just Hess s Law states that regardless of the multiple stages or steps of a reaction the total enthalpy change for the reaction is the sum of all changes
hess law of constant heat summation explain

hess law of constant heat summation explain
https://classroom-images.cdn.askfilo.com/classroom/1677680223031_dosudnva_3767263.jpg

Hess s Law Of Constant Heat Summation Thermodynamics NCERT
https://i.ytimg.com/vi/WBc2qryjENk/maxresdefault.jpg
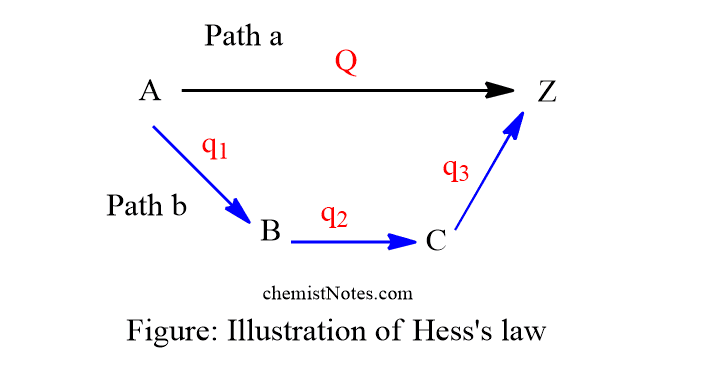
State And Explain Hess s Law Of Constant Heat Summation Chemistry Notes
https://chemistnotes.com/wp-content/uploads/2022/05/part-three.png
In 1840 Germain Hess a Swiss born Russian chemist and physician derived Hess s law of constant heat summation from a thermochemistry relationship for calculating the standard reaction enthalpy for Hess law of constant heat summation also known simply as Hess law is a relationship in physical chemistry and thermodynamics 1 named after Germain Hess a Swiss born Russian
Hess s law of heat summation states that if two or more thermochemical equations can be added together to give a final equation then the heats of reaction can also be added to Germain Henri Hess in 1840 discovered a very useful principle which is named for him The enthalpy of a given chemical reaction is constant regardless of the reaction happening in one
More picture related to hess law of constant heat summation explain

Hess Law Of Heat Summation
https://s2.studylib.net/store/data/005791651_1-66477adcf9af9841974f45293139c200-768x994.png
Solved Hess s Law Of Heat Summation States That If You Ca Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/b74/b74dbc29-20fe-47ce-8f01-be847a03090a/image

Class11 Hess s Law Of Constant Heat Summation Explanation In Telugu
https://i.ytimg.com/vi/_C-9Nf_6lc8/maxresdefault.jpg
Hess s Law which is also called Hess s Constant Heat Summation Law states the overall change in enthalpy for the solution can be given by the sum of all changes independent of the various Hess s law of heat summation states that if two or more thermochemical equations can be added together to give a final equation then the heats of reaction can also be added to
Hess s law of constant heat summation is based on the constancy of the total energy of the system before and after the reactions It is the same as the zeroth law of thermodynamics Hess s law of heat summation states that the total enthalpy change during a reaction is the same whether the reaction takes place in one step or in several steps For

Iii State Hess s Law Of Constant Heat Summation Illustrate With An
https://hi-static.z-dn.net/files/daf/00aa9b4c0c222fa3d146a331df237241.jpg

State And Explain Hess s Law Of Constant Heat Summation Give Its
https://d2rrqu68q7r435.cloudfront.net/images/6961597/9994c293-df53-4000-a6be-e01e5fefc517.jpg
hess law of constant heat summation explain - Hess s Law otherwise known as the law of constant heat summation is a fundamental principle in the field of chemistry This law asserts that the total enthalpy change
