half of 56 Half of 55 is the same as 55 divided by 2 which equals 27 5 The illustration uses long division to arrive at the decimal answer
From two half lives order of reaction can be determined as 1 If the values of half lives are decreasing then the order of the reaction is zero 2 If the half lives do not change then the order of the reaction is first 3 If the value of half life increasing with time then the order of the reaction is second Answer and Explanation 1 Learn how to halve a number by finding another number that adds to itself to give the original number The answer to what s half of 24 is 12 and you can see the explanation and examples on this web page
half of 56

half of 56
https://i2.wp.com/alittleperspective.com/wp-content/uploads/2015/09/isa-56-2-pixabay-9x.jpg
M16 Half Track 56 JPG Aircraft Of World War II WW2Aircraft Forums
https://ww2aircraft.net/forum/media/m16-half-track-56-jpg.29822/full

What Is Half Of 56 Updated For 2023
https://preview.redd.it/1awsj8qp5q971.jpg?width=640&crop=smart&auto=webp&s=9d7de06e9a0fdfb331ae6b2fc132aef8b2cd93b5
The radioactive isotope Cr 56 decays by beta emission If the mass of a sample of Chromium 56 decays from 76 0 micrograms to 9 50 micrograms in 17 7 minutes what is the half life of Chromium 56 The half life of indium 111 is t 1 2 2 805 days What is the decay constant of indium 111 The radioactive isotope 60Cu has a half life of 23 7 Learn how to divide a number into two equal parts and get half of 72 See the answer explanation and examples of basic division facts
For the reaction aAProducts A 0 4 4M and the first two half lives are 56 and 28 minutes respectively Calculate k without units A certain first order reaction is 73 complete in 65 seconds Calculate the half life for this reaction For a reaction aA to Products A 0 6 0 M and the first two half lives are 56 and 28 minutes For the reaction aAProducts A 0 4 4M and the first two half lives are 56 and 28 minutes respectively Calculate k without units The reaction aA to products is a first order reaction with a rate constant of 1 248 times 10 4 s 1 What fraction of reactant remains after 30 minutes
More picture related to half of 56

What Is Half Of 56 Go Easy Tips
https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhbcgiXLg1-YBBBOfmEVHTEK1Iv3wB6q_U5EzxZtbgMeHofUPEu4c62dv7GyNBbLT5CUg3A6IhQeJrFj-2zt0W8vs46jjIoYqiergAMQxvXXwmt4xiGf1DBOi5AEplgmUv7sOhCZMrnxCRaFg-pIBYuGibogAh0bWpPqfS41Rb9rlhFfyJSCEOvIYPCnQ/s1500/Image_1.jpg
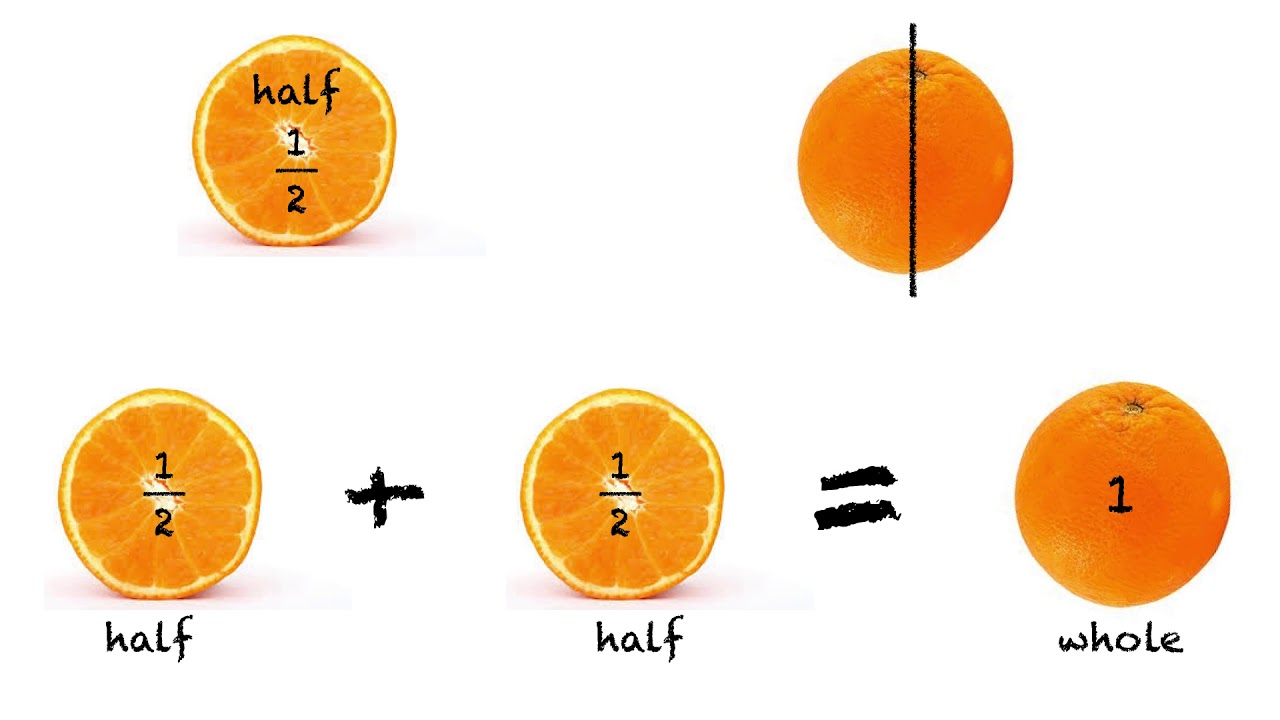
What S Half Of 56 Update Countrymusicstop
https://i.ytimg.com/vi/5TDC6oU8ht4/maxresdefault.jpg

File Half Dome10 jpg Wikipedia
http://upload.wikimedia.org/wikipedia/commons/a/ab/Half_Dome10.jpg
The half life of chromium is 28 days If the original sample contained 510 grams how much chromium would remain after 56 days Isotope chromium 51 has a half life of 28 days If the original sample had a mass of 28 0 grams what mass of chromium 51 sample remains after 84 days The half life of iodine 125 is 60 days The half life is 990 seconds or about 16 minutes Example Problem 2 Identifying Half Life Given the Rate Constant Sulfuryl chloride is a foul smelling liquid
[desc-10] [desc-11]

What Is Half Of 56 Go Easy Tips
https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEiffIjxhbfqO7RfMOm42_ItKHQP-QCjDyMjPDzmwG2ULfaQ5nNNVuqiMSQH67qd0D2N-bRHDa73W2wce4vD5dE4pkdjlqQtNpuRy0sv6b4bM3p05qycJSZi2PxoCEA8kX0dMe_17Fnu1aLL6JTuAuPbiOT63yhut9qEIicaZZ2Eo28znxsAXoZq8jcW9g/s600/images.png

5 56 SHOOTING PHOTO
https://marine-comby.book.fr/files/1/186675/g_30_yl2bT2ogWk.jpg
half of 56 - [desc-13]
