Gibbs Free Energy Worksheet GIBB S FREE ENERGY PROBLEMS WORKSHEET Answer feel free to question my math Also sig figs The hydrogenation of ethene gas under standard conditions T 298 15 K shows a decrease in disorder S 0 1207 kJ mol K during an exothermic reaction H 136 9 kJ mol
1 Calculate the change in entropy of a large vat of molten copper when 50 J of energy is removed reversible from it as heat at 1100 C 2 Calculate the change in entropy of 1 0 L of water at 0 C when it absorbs 235 J of energy from a heater b If the 1 0 L of water is at 99 C what is its entropy change S7 E 2a The freezing of water is a decrease in entropy so it has the smallest Delta S because solids have less entropy than liquids The other two options represent an increase in Entropy The sublimation of ice is a large increase in Entropy because gas has more Entropy than solids The sublimation of ice to gas is more of an increase than
Gibbs Free Energy Worksheet

Gibbs Free Energy Worksheet
https://d2j6dbq0eux0bg.cloudfront.net/images/15443271/959738933.jpg
Gibbs Free Energy Worksheet
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/5d918962a31ed0bedc2fa5cd17e53932/thumb_1200_1697.png

Gibbs Free Energy Definition Equation Unit And Example
https://www.chemistrylearner.com/wp-content/uploads/2022/01/Gibbs-Free-Energy-Graph.jpg
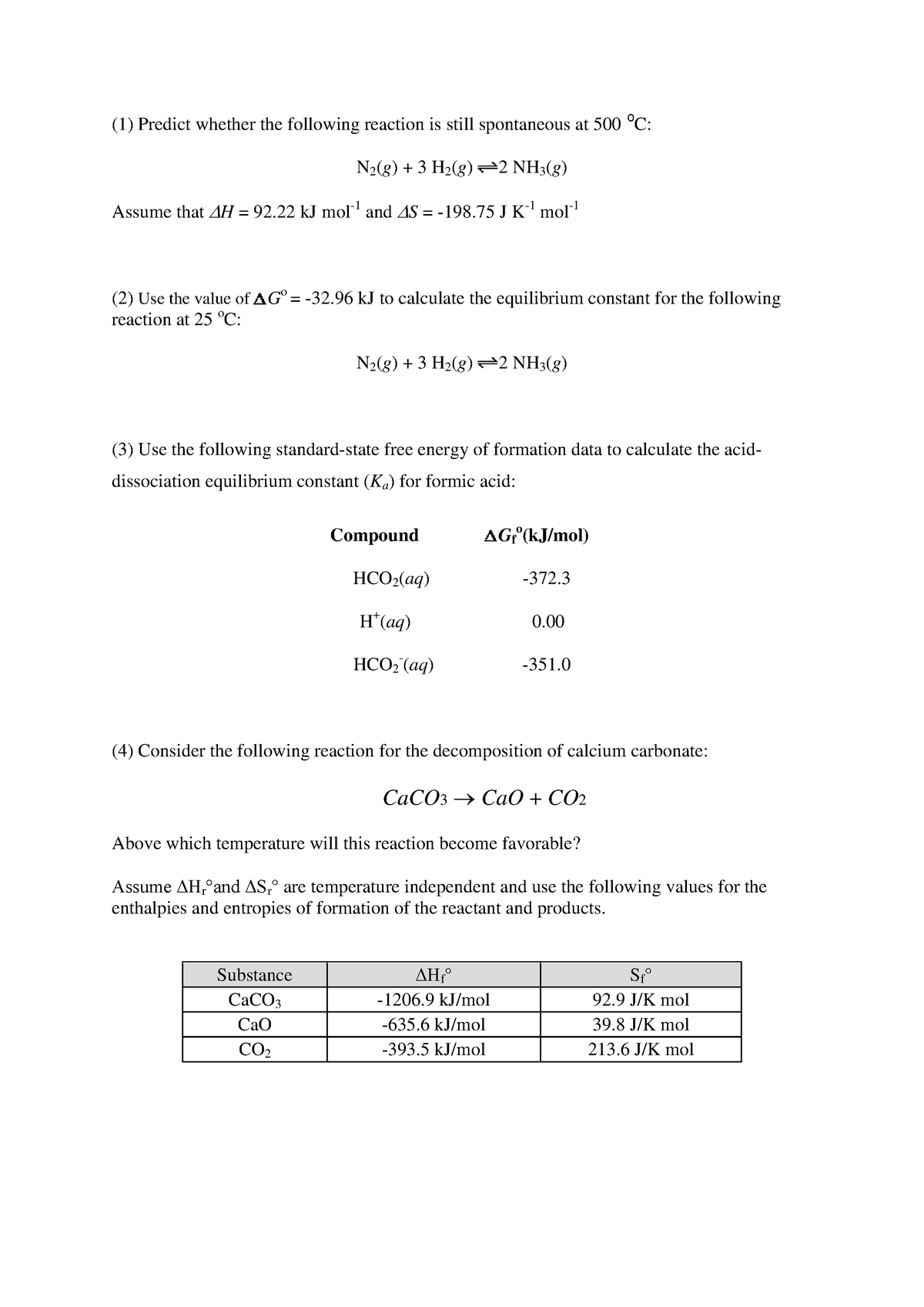
1 PRACTICE PROBLEM At 25 C the G of the following reaction is 1169 2 kJ mol Th s O 2 g ThO 2 s Determine if the reaction is spontaneous at 25 C 2 PRACTICE PROBLEM Consider the following reaction 2 Sn s O 2 g 2 SnO s H 561 42 kJ mol 134 18 kcal mol Is the reaction spontaneous at all temperatures 3 PRACTICE PROBLEM Gibbs Free Energy Practice Problems 1 PRACTICE PROBLEM At 25 C the K a for HF is 7 2 10 4 Determine G at equilibrium 2 PRACTICE PROBLEM The reactants and products in the gaseous phase for the following reactions are present at 1 atm partial pressure In the forward direction which reactions proceed spontaneously
Spontaneity is determined by the overall change in Gibb s free energy G i e energy available to do work A reaction is spontaneous when G 0 and is nonspontaneous when G 0 Gibb s free energy can be calculated from enthalpy change H and entropy change S randomness or degree of disorder Exercise 18 6 1a Balance the following equations Calculate the change of the Gibbs free energy for the reaction at 25 C where standard free energy of formation of C 2 H 4 g H 2 O g C 2 H 5 OH l are 68 229 175 kJ mol respectively C2H4 g H2O g C2H5OH l What is the change of entropy if S 0 of C 2 H 4 g H 2 O g C 2 H
More picture related to Gibbs Free Energy Worksheet
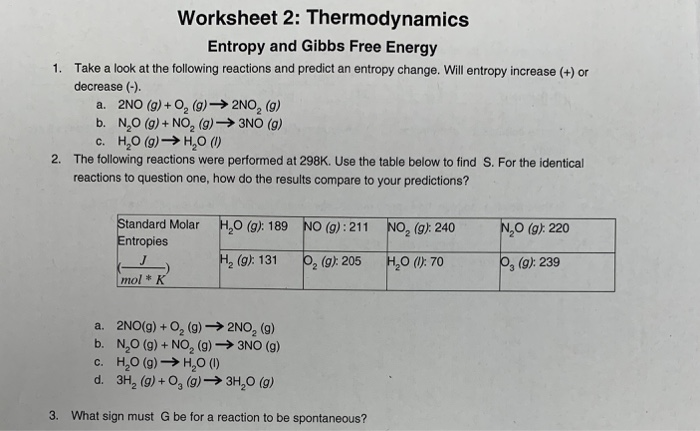
Solved Worksheet 2 Thermodynamics Entropy And Gibbs Free Chegg
https://media.cheggcdn.com/media/de8/de82b24e-44fc-40a3-ab37-d385e012ec7f/image.png

Gibbs Free Energy Worksheet Answers worksheet
https://i.pinimg.com/originals/fe/0a/78/fe0a78fc50e3cb8b0652c8c18d5081e1.gif

AP WORKSHEET 9c Entropy Enthalpy And Gibbs Free Energy
https://s3.studylib.net/store/data/009037472_1-da7ca380f7683bd1335217af0d380c70-768x994.png
At constant temperature and pressure the change in Gibbs free energy is defined as G H T S When G is negative a process will proceed spontaneously and is referred to as exergonic The spontaneity of a process can depend on the temperature Spontaneous processes 21 Multiple Choice Fe 2 O 3 s 3 H 2 g 2 Fe s 3 H 2 O g is a redox reaction What would be its Gibbs Free energy change under standard conditions Is the reaction spontaneous at 25 C 21 Learn Gibbs Free Energy Calculations with free step by step video explanations and practice problems by experienced tutors
We can answer this question by defining a new quantity known as the Gibbs free energy G of the system which reflects the balance between these forces The Gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system G H TS The standard free energy change can be calculated from the definition of free energy if the standard enthalpy and entropy changes are known using Equation 18 8 7 G H T S If S and H for a reaction have the same sign then the sign of G depends on the relative magnitudes of the H and T S terms

Gibbs Free Energy Practice Problems
https://s3.studylib.net/store/data/008857934_1-5ac4d17e9791bf9019f9a0eb69b8ecbd.png

Gibbs Free Energy Worksheet Nidecmege EnergyWorksheet
https://i0.wp.com/www.energyworksheet.com/wp-content/uploads/2022/10/gibbs-free-energy-worksheet-nidecmege.png?fit=574%2C590&ssl=1
Gibbs Free Energy Worksheet - 1 PRACTICE PROBLEM At 25 C the G of the following reaction is 1169 2 kJ mol Th s O 2 g ThO 2 s Determine if the reaction is spontaneous at 25 C 2 PRACTICE PROBLEM Consider the following reaction 2 Sn s O 2 g 2 SnO s H 561 42 kJ mol 134 18 kcal mol Is the reaction spontaneous at all temperatures 3 PRACTICE PROBLEM