formula units of mgcl2 Magnesium chloride is an inorganic compound with the formula MgCl2 It forms hydrates MgCl2 nH2O where n can range from 1 to 12 These salts are colorless or white solids that are highly soluble in water These compounds and their solutions both of which occur in nature have a variety of practical uses Anhydrous magnesium chloride is the principal precursor to magnesium metal
Formula unit mass is used to calculate the mass of ionic compounds It is the sum of the atomic masses of all the ions in the compound Hence the formula unit mass of MgCl 2 atomic The formula MgCl2 tells us that for every unit of MgCl2 there are 2 units of Cl The way we express this numerically where our units are moles as specified by this problem is with a ratio 2 mol Cl 1 mol MgCl2 or 1 mol MgCl2 2 mol Cl
formula units of mgcl2

formula units of mgcl2
https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/120/12050926.jpeg

Solved How Many Moles Of MgCl2 Are Present In 60 0 ML Of Chegg
https://media.cheggcdn.com/study/42b/42b24549-da82-4232-95e1-f6a590d31b59/image.jpg

Find The Ratio By Mass Of The Following Compounds A CaO B MgCl2 C
https://hi-static.z-dn.net/files/d94/7f599d2d6c622482df7084e4196c74ce.jpg
Explanation Magnesium chloride is an ionic compound with an Mg2 ion and two Cl ions in each formula unit Thus 1 2 1024 formula units 1 mol MgCl2 6 023 1023 You also know that in order for your sample to contain 1 mole of magnesium chloride it must contain 6 022 10 23 formula units of magnesium chloride this is given
How many formula units make up 39 8 g of magnesium chloride MgCl2 Calculate the number of moles of Cl atoms in 1 81 1024 formula units of magnesium chloride MgCl2 See an expert written answer What is Magnesium Chloride MgCl 2 can be extracted from seawater or brine and is chemically named Magnesium Chloride Magnesium Chloride is also called Magnesium dichloride or
More picture related to formula units of mgcl2

Molar Mass Molecular Weight Of MgCl2 6H2O Magnesium Chloride
https://i.ytimg.com/vi/p8RZ_f0e7Fc/maxresdefault.jpg

pingl Par Pavel Pilovets Sur ART Character Vehicle Formule 1 Photo
https://i.pinimg.com/originals/5a/75/cd/5a75cd53a0b682360b7cb5fdac5ccc7f.jpg

Formula 1 F1 Wallpaper Hd F1 Drivers Sport Man Comfort Zone Tik
https://i.pinimg.com/originals/e5/70/6f/e5706fce23829318dab66cdd606563c1.jpg
At the heart of magnesium chloride s usefulness is its unique set of chemical and physical properties It is an ionic compound composed of one magnesium ion Mg 2 and two chloride Magnesium chloride Formula Cl 2 Mg Molecular weight 95 211 CAS Registry Number 7786 30 3 Information on this page Notes Other data available Gas phase thermochemistry data
Magnesium chloride has a molar mass of 95 211 g mol 1 which means that every mole of magnesium chloride has a mass of 95 211 g This means that your sample will The formula mass for MgCl2 magnesium chloride is calculated by adding the atomic masses of magnesium Mg and chlorine Cl The Atomic Mass of Mg is 24 31 g mol

Purple Formula 1 Car
https://pics.craiyon.com/2023-09-10/010cb40907d74c3bbae874c8b5fee6d2.webp
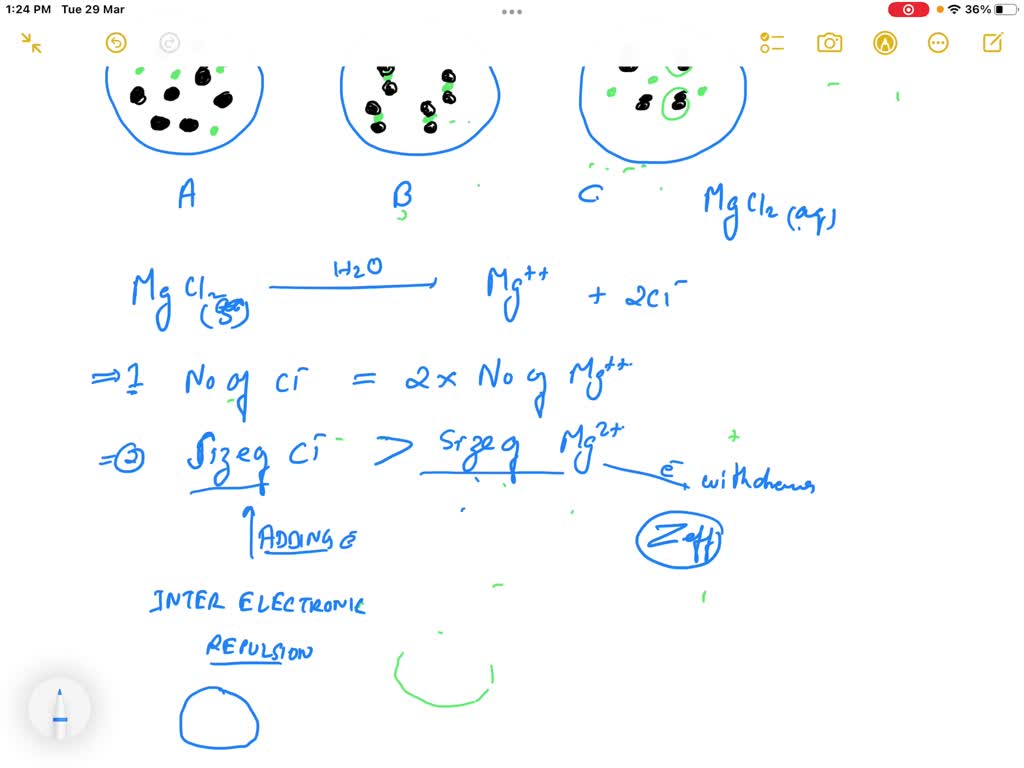
SOLVED Which Of The Diagrams In The Figure Best Represents An Aqueous
https://cdn.numerade.com/ask_previews/90652a50-d426-4dd2-9cea-840245441dbb_large.jpg
formula units of mgcl2 - You also know that in order for your sample to contain 1 mole of magnesium chloride it must contain 6 022 10 23 formula units of magnesium chloride this is given